Case Report: Afatinib Treatment in a Patient With NSCLC Harboring a Rare EGFR Exon 20 Mutation
- PMID: 33575211
- PMCID: PMC7871906
- DOI: 10.3389/fonc.2020.593852
Case Report: Afatinib Treatment in a Patient With NSCLC Harboring a Rare EGFR Exon 20 Mutation
Abstract
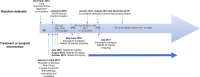
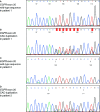
Unlike most other primary epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC), exon 20 insertions, comprising approximately 4% to 10% of all EGFR mutations, are generally considered to be resistant to EGFR tyrosine kinase inhibitors (TKIs). However, EGFR exon 20 insertions are structurally and pharmacologically heterogeneous, with variability in their position and size having implications for response to different EGFR TKIs. The second-generation ErbB family blocker, afatinib, is approved for the first-line treatment of EGFR mutation-positive NSCLC and has been shown to have a broad inhibitory profile against common and uncommon EGFR mutations. Here, we describe a patient with bilateral multifocal lung adenocarcinoma harboring a very rare EGFR exon 20 insertion (c.2317_2319dup3; p.H773dup), who has been receiving treatment with afatinib for 4.5 years. To our knowledge, this is the first report describing long-term benefit for a patient treated with afatinib with this rare exon 20 insertion. We are aware of two further cases with this rare EGFR mutation. One patient, also reported here, has early-stage lung adenocarcinoma and has not yet received systemic therapy for NSCLC. The other patient received afatinib in the context of a global compassionate use program and had progressive disease. Our findings may be of clinical relevance for patients carrying tumors with this rare mutation as epidemiological evidence suggests that p.H773dup may function as a driver mutation in NSCLC. Together with previous preclinical and clinical evidence for the activity of afatinib against certain EGFR exon 20 insertions, these findings warrant further investigation.
Keywords: EGFR mutation; H773dup; NSCLC; afatinib; exon 20 insertion; long-term response; uncommon mutation.
Copyright © 2021 Zöchbauer-Müller, Kaserer, Prosch, Cseh, Solca, Bauer and Müllauer.
Conflict of interest statement
SZM reports advisory council or committee relationship with Boehringer Ingelheim, Roche, MSD, BMS, Takeda, and AstraZeneca, honoraria from Bayer and Pfizer, and grants or funds from MSD. HP reports advisory council or committee relationship with Boehringer Ingelheim, Roche, MSD, and honoraria from Boehringer Ingelheim, Roche, MSD, BMS, and AstraZeneca. AC, FS, and MB report employment with Boehringer Ingelheim. LM reports consulting fees (advisory boards) from Boehringer Ingelheim. The authors declare that this case report received funding for medical writing assistance from Boehringer Ingelheim. The funder was involved in the conception and design, analysis and interpretation of data, knowledge generation from molecular data, the writing and reviewing of this article, the final approval, and the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

