Confined Electrochemiluminescence Generation at Ultra-High-Density Gold Microwell Electrodes
- PMID: 33575249
- PMCID: PMC7870482
- DOI: 10.3389/fchem.2020.630246
Confined Electrochemiluminescence Generation at Ultra-High-Density Gold Microwell Electrodes
Abstract
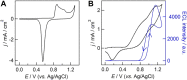
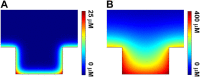
Electrochemiluminescence (ECL) imaging analysis based on the ultra-high-density microwell electrode array (UMEA) has been successfully used in biosensing and diagnostics, while the studies of ECL generation mechanisms with spatial resolution remain scarce. Herein we fabricate a gold-coated polydimethylsiloxane (PDMS) UMEA using electroless deposition method for the visualization of ECL reaction process at the single microwell level in conjunction with using microscopic ECL imaging technique, demonstrating that the microwell gold walls are indeed capable of enhancing the ECL generation. For the classical ECL system involving tris(2,2'-bipyridyl)ruthenium (Ru(bpy)3 2+) and tri-n-propylamine (TPrA), the ECL image of a single microwell appears as a surface-confined ring, indicating the ECL intensity generated inside the well is much stronger than that on the top surface of UMEA. Moreover, at a low concentration of Ru(bpy)3 2+, the ECL image remains to be ring-shaped with the increase of exposure time, because of the limited lifetime of TPrA radical cations TPrA+•. In combination with the theoretical simulation, the ring-shaped ECL image is resolved to originate from the superposition effect of the mass diffusion fields at both microwell wall and bottom surfaces.
Keywords: confinement; electrochemiluminescence; imaging; microwell electrode array; reaction mechanisms.
Copyright © 2021 Ding, Zhou, Guo and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

