Analysis of COVID-19-Related RT-qPCR Test Results in Hungary: Epidemiology, Diagnostics, and Clinical Outcome
- PMID: 33575263
- PMCID: PMC7870862
- DOI: 10.3389/fmed.2020.625673
Analysis of COVID-19-Related RT-qPCR Test Results in Hungary: Epidemiology, Diagnostics, and Clinical Outcome
Abstract
Background: Effective testing is an essential tool for controlling COVID-19. We aimed to analyse the data from first-wave PCR test results in Hungary's Southern Transdanubian region to improve testing strategies. Methods: We performed a retrospective analysis of all suspected COVID-19 cases between 17 March and 8 May 2020, collecting epidemiological, demographic, clinical and outcome data (ICU admission and mortality) with RT-qPCR test results. Descriptive and comparative statistical analyses were conducted. Results: Eighty-six infections were confirmed among 3,657 tested patients. There was no difference between the positive and negative cases in age and sex distribution; however, ICU admission (8.1 vs. 3.1%, p = 0.006) and in-hospital mortality (4.7 vs. 1.6%, p = 0.062) were more frequent among positive cases. Importantly, none of the initially asymptomatic patients (n = 20) required ICU admission, and all survived. In almost all cases, if the first test was negative, second and third tests were performed with a 48-h delay for careful monitoring of disease development. However, the positive hit rate decreased dramatically with the second and third tests compared to the first (0.3 vs. 2.1%, OR = 0.155 [0.053-0.350]). Higher E-gene copy numbers were associated with a longer period of PCR positivity. Conclusion: In our immunologically naïve suspected COVID-19 population, coronavirus infection increased the need for intensive care and mortality by 3-4 times. In the event of the exponential phase of the pandemic involving a bottleneck in testing capacity, a second or third test should be reconsidered to diagnose more coronavirus infections.
Keywords: COVID-19; PCR diagnostics; SARS-CoV-2; epidemiology; surveillance; testing.
Copyright © 2021 Gombos, Földi, Kiss, Herczeg, Gyenesei, Geiger, Csabai, Futács, Nagy, Miseta, Somogyi, Hegyi and Szentesi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
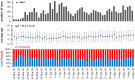

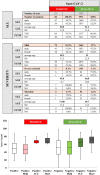

References
-
- Johns-Hopkins-Coronavirus-Resource-Center (2020). Available online at: https://coronavirus.jhu.edu/map.html (accessed November 1, 2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

