Age-specific microbiota in altering host inflammatory and metabolic signaling as well as metabolome based on the sex
- PMID: 33575288
- PMCID: PMC7867716
- DOI: 10.21037/hbsn-20-671
Age-specific microbiota in altering host inflammatory and metabolic signaling as well as metabolome based on the sex
Abstract
Background: Metabolism is sex-different, and the direct link between gut microbiota and aging-associated metabolic changes needs to be established in both sexes.
Methods: Gene expression, metabolic and inflammatory signaling, gut microbiota profile, and metabolome were studied during aging and after fecal microbiota transplantation (FMT) in mice of both sexes.
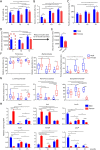
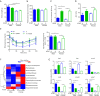
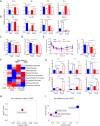
Results: Our data revealed young female mice and aged male mice were the most insulin sensitive and resistant group, respectively. In addition, aging reduced sex difference in insulin sensitivity. Such age- and sex-dependent metabolic phenotypes were accompanied by shifted gut microbiota profile and altered abundance of bacterial genes that produce butyrate, propionate, and bile acids. After receiving feces from the aged males (AFMT), the most insulin-resistant group, recipients of both sexes had increased hepatic inflammation and serum endotoxin. However, AFMT only increased insulin resistance in female mice and abolished sex difference in insulin sensitivity. Additionally, such changes were accompanied by narrowed sex difference in metabolome. Metabolomics data revealed that age-associated insulin resistance in males was accompanied by increased sugar alcohols and dicarboxylic acids as well as reduced aromatic and branched-chain amino acids. Further, receiving feces from the young females (YFMT), the most insulin-sensitive group, reduced body weight and fasting blood glucose in male recipients and improved insulin sensitivity in females, leading to enhanced sex differences in insulin sensitivity and metabolome.
Conclusions: Aging systemically affected inflammatory and metabolic signaling based on the sex. Gut microbiome is age and sex-specific, which affects inflammation and metabolism in a sex-dependent manner.
Keywords: Aging; fecal transplantation; insulin sensitivity; metabolome; microbiome.
2021 Hepatobiliary Surgery and Nutrition. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/hbsn-20-671). Dr. YJYW serves as an unpaid editorial board member of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
