Novel progresses of chimeric antigen receptor (CAR) T cell therapy in multiple myeloma
- PMID: 33575314
- PMCID: PMC7867711
- DOI: 10.21037/sci-2020-029
Novel progresses of chimeric antigen receptor (CAR) T cell therapy in multiple myeloma
Abstract
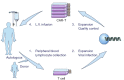
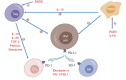
Multiple myeloma (MM) is a malignant proliferative disease of plasma cells, which leads to suppressed hematopoietic and osteolytic diseases. Despite the use of traditional chemotherapy, hematopoietic stem cell transplantation (HSCT) and targeted drugs, MM still cannot be completely cured. In recent years, chimeric antigen receptor (CAR) T cells have revolutionized immunotherapy and cancer treatment. The great success of CAR-T cells in leukemia and lymphoma has promoted its development in MM. The primary requisite for developing clinically effective CAR-T cells suitable for MM is to identify the appropriate targets. In early clinical trials, CAR-T cells targeting B-cell maturation antigen (BCMA) have shown significant anti-MM activity. Currently popular targets in clinical research and preclinical research include CD138, CD38, CS1, CD19, κ light chain, CD56, CD44v6, Lewis Y, NY-ESO-1, CD229, etc. Common toxicities such as cytokine release syndrome (CRS) and neurotoxicity also occur but controllable. MM cells are mainly localized in bone marrow, therefore, the bone marrow microenvironment has a significant effect on the therapeutic effect of CAR-T cells. Targeting both MM cells and the bone marrow microenvironment is currently the most promising treatment. In this review, we provide a comprehensive overview of CAR-T cell therapy in MM, as well as outline potential targets and methods that can overcome local immunosuppression and improve the efficacy of CAR-T cells.
Keywords: B-cell maturation antigen (BCMA); Chimeric antigen receptor T cells (CAR-T cells); microenvironment; multiple myeloma (MM); target.
2021 Stem Cell Investigation. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-029). Dr. HH serves as an unpaid associate editor of Stem Cell Investigation. The other authors have no conflicts of interest to declare.
Figures
Similar articles
-
CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond.Vaccines (Basel). 2023 Nov 16;11(11):1721. doi: 10.3390/vaccines11111721. Vaccines (Basel). 2023. PMID: 38006053 Free PMC article. Review.
-
Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen.Front Immunol. 2019 Jul 16;10:1613. doi: 10.3389/fimmu.2019.01613. eCollection 2019. Front Immunol. 2019. PMID: 31379824 Free PMC article. Review.
-
CAR T-cell therapy for multiple myeloma: state of the art and prospects.Lancet Haematol. 2021 Jun;8(6):e446-e461. doi: 10.1016/S2352-3026(21)00057-0. Lancet Haematol. 2021. PMID: 34048683 Review.
-
Hematologic Malignancies: Plasma Cell Disorders.Am Soc Clin Oncol Educ Book. 2017;37:561-568. doi: 10.1200/EDBK_175546. Am Soc Clin Oncol Educ Book. 2017. PMID: 28561703 Review.
-
Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis.Cytotherapy. 2017 Jul;19(7):861-866. doi: 10.1016/j.jcyt.2017.03.077. Epub 2017 May 5. Cytotherapy. 2017. PMID: 28483281
Cited by
-
Chimeric Antigen Receptor T-Cell Therapy in Lung Cancer: Potential and Challenges.Front Immunol. 2021 Nov 1;12:782775. doi: 10.3389/fimmu.2021.782775. eCollection 2021. Front Immunol. 2021. PMID: 34790207 Free PMC article. Review.
-
Chimeric Antigen Receptor-Engineered Natural Killer (CAR NK) Cells in Cancer Treatment; Recent Advances and Future Prospects.Stem Cell Rev Rep. 2021 Dec;17(6):2081-2106. doi: 10.1007/s12015-021-10246-3. Epub 2021 Sep 2. Stem Cell Rev Rep. 2021. PMID: 34472037 Free PMC article. Review.
-
Bispecific T Cell Engagers for the Treatment of Multiple Myeloma: Achievements and Challenges.Cancers (Basel). 2021 Jun 8;13(12):2853. doi: 10.3390/cancers13122853. Cancers (Basel). 2021. PMID: 34201007 Free PMC article. Review.
-
Targets for CAR Therapy in Multiple Myeloma.Int J Mol Sci. 2025 Jun 24;26(13):6051. doi: 10.3390/ijms26136051. Int J Mol Sci. 2025. PMID: 40649828 Free PMC article. Review.
-
Characterization of the Therapeutic Effects of Novel Chimeric Antigen Receptor T Cells Targeting CD38 on Multiple Myeloma.Front Oncol. 2021 Aug 26;11:703087. doi: 10.3389/fonc.2021.703087. eCollection 2021. Front Oncol. 2021. PMID: 34513682 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
