Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital
- PMID: 33575418
- PMCID: PMC7863868
- DOI: 10.1093/ofid/ofaa610
Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital
Abstract
Background: Due to unprecedented shortages in N95 filtering facepiece respirators, healthcare systems have explored N95 reprocessing. No single, full-scale reprocessing publication has reported an evaluation including multiple viruses, bacteria, and fungi along with respirator filtration and fit.
Methods: We explored reprocessing methods using new 3M 1860 N95 respirators, including moist (50%-75% relative humidity [RH]) heat (80-82°C for 30 minutes), ethylene oxide (EtO), pulsed xenon UV-C (UV-PX), hydrogen peroxide gas plasma (HPGP), and hydrogen peroxide vapor (HPV). Respirator samples were analyzed using 4 viruses (MS2, phi6, influenza A virus [IAV], murine hepatitis virus [MHV)]), 3 bacteria (Escherichia coli, Staphylococcus aureus, Geobacillus stearothermophilus spores, and vegetative bacteria), and Aspergillus niger. Different application media were tested. Decontaminated respirators were evaluated for filtration integrity and fit.
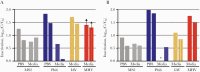
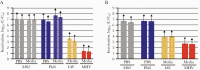
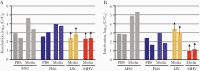
Results: Heat with moderate RH most effectively inactivated virus, resulting in reductions of >6.6-log10 MS2, >6.7-log10 Phi6, >2.7-log10 MHV, and >3.9-log10 IAV and prokaryotes, except for G stearothermohphilus. Hydrogen peroxide vapor was moderately effective at inactivating tested viruses, resulting in 1.5- to >4-log10 observable inactivation. Staphylococcus aureus inactivation by HPV was limited. Filtration efficiency and proper fit were maintained after 5 cycles of heat with moderate RH and HPV. Although it was effective at decontamination, HPGP resulted in decreased filtration efficiency, and EtO treatment raised toxicity concerns. Observed virus inactivation varied depending upon the application media used.
Conclusions: Both moist heat and HPV are scalable N95 reprocessing options because they achieve high levels of biological indicator inactivation while maintaining respirator fit and integrity.
Keywords: N95; decontamination; inactivation; reprocessing; virus.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Centers for Disease Control and Prevention. NIOSH-Approved N95 Particulate Filtering Facepiece Respirators - 3M Suppliers List Available at: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html. Accessed 26 April 2020.
-
- Centers for Disease Control and Prevention. Infection Control: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommen.... Accessed 26 April 2020.
-
- Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg 2012; 56:92–101. - PubMed
-
- Heimbuch BK, Wallace WH, Kinney K, et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control 2011; 39:e1–9. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

