[Treatment of nonmetastatic CRPC : Androgen receptor inhibition as new treatment standard in nonmetastatic castration-resistant prostate cancer with high risk of metastasis]
- PMID: 33575824
- PMCID: PMC8208922
- DOI: 10.1007/s00120-021-01473-0
[Treatment of nonmetastatic CRPC : Androgen receptor inhibition as new treatment standard in nonmetastatic castration-resistant prostate cancer with high risk of metastasis]
Abstract
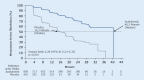
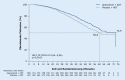
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC or M0CRPC) at high risk of progression (defined as prostate-specific antigen [PSA] doubling time ≤ 10 months), new androgen receptor inhibitors (ARI) in combination with continued androgen deprivation therapy (ADT) are considered the new standard of care. Apalutamide, enzalutamide, and darolutamide have been approved on the basis of improved metastasis-free survival (MFS) in the respective large pivotal studies SPARTAN, PROSPER and ARAMIS and now, with a longer follow-up period, were able to show also a statistically significant and clinically relevant overall survival advantage compared to placebo plus ADT. The data available to date indicate that all three ARIs are comparably effective, accompanied by good tolerability. Moreover, the generally good quality of life of this patient population, who usually has no tumor-related symptoms, was maintained. Comparative head-to-head trials of the three approved substances are not available yet.
Bei Patienten mit nicht-fernmetastasiertem kastrationsresistentem Prostatakarzinom (nmCRPC oder M0CRPC) und hohem Progressionsrisiko (definiert als PSA-Verdopplungszeit ≤ 10 Monate) stellen selektive Androgenrezeptorinhibitoren (ARI) der neuen Generation in Kombination mit fortgeführter Androgendeprivationstherapie (ADT) den neuen Therapiestandard dar. Zugelassen sind derzeit Apalutamid, Enzalutamid und Darolutamid, die im Vorfeld bereits in den jeweiligen großen Phase-III-Zulassungsstudien SPARTAN, PROSPER und ARAMIS ihren primären Endpunkt metastasenfreies Überleben (MFS) erreicht hatten und jetzt nach längerer Nachbeobachtungszeit auch einen statistisch signifikanten und klinisch relevanten Gesamtüberlebensvorteil gegenüber Placebo plus ADT zeigen konnten. Die bisherige Datenlage weist auf eine vergleichbar gute Effektivität aller drei Substanzen bei insgesamt guter Verträglichkeit hin. Auch die generell gute Lebensqualität dieser Patientenpopulation, die in der Regel noch keine tumorbedingten Symptome aufweist, konnte erhalten werden. Direkt vergleichende Head-to-head-Studien der drei zugelassenen Substanzen liegen bislang nicht vor.
Keywords: Androgen deprivation therapy; Apalutamide; Darolutamide; Enzalutamlide; Prostate cancer.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous