Population pharmacokinetics and pharmacodynamics of Tranexamic acid in women undergoing caesarean delivery
- PMID: 33576009
- PMCID: PMC8355246
- DOI: 10.1111/bcp.14767
Population pharmacokinetics and pharmacodynamics of Tranexamic acid in women undergoing caesarean delivery
Abstract
Aims: The population pharmacokinetics (PK) and pharmacodynamics (PD) of tranexamic acid (TXA) have not been studied to prevent postpartum haemorrhage (PPH) in pregnant women. It is unclear which TXA dose assures sufficient PPH prevention. This study investigated population PK/PD of TXA in pregnant women who underwent caesarean delivery to determine the optimal prophylactic doses of TXA for future studies.
Methods: We analysed concentration (PK) and maximum lysis (PD) data from 30 pregnant women scheduled for caesarean delivery who received 5, 10 or 15 mg/kg of TXA intravenously using population approach.
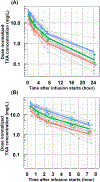
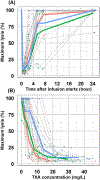
Results: TXA PK was best described by a two-compartment model with first-order elimination and the following parameters: clearance (between-subject variability) of 9.4 L/h (27.7%), central volume of 10.1 L (47.4%), intercompartmental clearance of 22.4 L/h (66.7%), peripheral volume of 14.0 L (13.1%) and additive error of 1.4 mg/L. The relationship between TXA concentration and maximum lysis was characterized by a sigmoid Emax model with baseline lysis of 97%, maximum inhibition of 89%, IC50 of 6.0 mg/L (65.3%), hill factor of 8.5 (86.3%) and additive error of 7.3%. Simulations demonstrated that 500 and 650 mg of TXA maintained therapeutic targets for 30 minutes and 1 hour, respectively, in 90% of patients.
Conclusion: This is the first population PK and PD study of TXA in pregnant women undergoing caesarean delivery. Our analysis suggests that a 650 mg dose provides adequate PPH prophylaxis up to 1 hour, which is less than the currently used 1000 mg of TXA in pregnant women.
Keywords: TXA; population pharmacodynamics; population pharmacokinetics; post-partum haemorrhage; prophylaxis.
© 2021 British Pharmacological Society.
Conflict of interest statement
Conflict of Interest
Jogarao V.S. Gobburu is a co-founder of Pumas-AI that commercializes Pumas software.
Figures

Similar articles
-
WOMAN-PharmacoTXA trial: Study protocol for a randomised controlled trial to assess the pharmacokinetics and pharmacodynamics of intramuscular, intravenous and oral administration of tranexamic acid in women giving birth by caesarean section.Wellcome Open Res. 2021 Jun 16;6:157. doi: 10.12688/wellcomeopenres.16884.1. eCollection 2021. Wellcome Open Res. 2021. PMID: 34250266 Free PMC article.
-
Alternative routes for tranexamic acid treatment in obstetric bleeding (WOMAN-PharmacoTXA trial): a randomised trial and pharmacological study in caesarean section births.BJOG. 2023 Sep;130(10):1177-1186. doi: 10.1111/1471-0528.17455. Epub 2023 Apr 5. BJOG. 2023. PMID: 37019443 Clinical Trial.
-
Tranexamic acid by the intramuscular or intravenous route for the prevention of postpartum haemorrhage in women at increased risk: a randomised placebo-controlled trial (I'M WOMAN).Trials. 2023 Dec 3;24(1):782. doi: 10.1186/s13063-023-07687-1. Trials. 2023. PMID: 38044460 Free PMC article. Clinical Trial.
-
Meta-analysis: the prophylactic use of tranexamic acid to reduce blood loss during caesarean delivery.Ir J Med Sci. 2025 Feb;194(1):311-322. doi: 10.1007/s11845-024-03834-y. Epub 2024 Dec 9. Ir J Med Sci. 2025. PMID: 39652279 Review.
-
The role of tranexamic acid in the management of postpartum haemorrhage.Best Pract Res Clin Anaesthesiol. 2022 Dec;36(3-4):411-426. doi: 10.1016/j.bpa.2022.08.004. Epub 2022 Aug 31. Best Pract Res Clin Anaesthesiol. 2022. PMID: 36513435 Review.
Cited by
-
Traumatic bleeding and mortality in mice are intensified by iron deficiency anemia and can be rescued with tranexamic acid.Res Pract Thromb Haemost. 2024 Aug 8;8(6):102543. doi: 10.1016/j.rpth.2024.102543. eCollection 2024 Aug. Res Pract Thromb Haemost. 2024. PMID: 39286605 Free PMC article.
-
WOMAN-PharmacoTXA trial: Study protocol for a randomised controlled trial to assess the pharmacokinetics and pharmacodynamics of intramuscular, intravenous and oral administration of tranexamic acid in women giving birth by caesarean section.Wellcome Open Res. 2021 Jun 16;6:157. doi: 10.12688/wellcomeopenres.16884.1. eCollection 2021. Wellcome Open Res. 2021. PMID: 34250266 Free PMC article.
-
Tranexamic acid dose-response relationship for antifibrinolysis in postpartum haemorrhage during Caesarean delivery: TRACES, a double-blind, placebo-controlled, multicentre, dose-ranging biomarker study.Br J Anaesth. 2022 Dec;129(6):937-945. doi: 10.1016/j.bja.2022.08.033. Epub 2022 Oct 13. Br J Anaesth. 2022. PMID: 36243576 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Curative Tranexamic Acid in Parturients Undergoing Cesarean Delivery.Pharmaceutics. 2022 Mar 6;14(3):578. doi: 10.3390/pharmaceutics14030578. Pharmaceutics. 2022. PMID: 35335955 Free PMC article.
-
Tranexamic acid for the prevention and treatment of postpartum hemorrhage in resource-limited settings: a literature review.Ann Med Surg (Lond). 2023 Nov 27;86(1):353-360. doi: 10.1097/MS9.0000000000001560. eCollection 2024 Jan. Ann Med Surg (Lond). 2023. PMID: 38222769 Free PMC article. Review.
References
-
- Shakur H, Roberts I, Fawole B, et al.Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. doi:10.1016/S0140-6736(17)30638-4 - DOI - PMC - PubMed
-
- World Health Organization (WHO). Updated WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage: Highlights and Key Messages from the World Health Organization’s 2017 Global Recommendation. 2017;(October):5. - PubMed
-
- CykloKapron package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019281s030lbl.pdf.2012;(11):2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

