Cognitive decline in amyotrophic lateral sclerosis: Neuropathological substrate and genetic determinants
- PMID: 33576076
- PMCID: PMC8412113
- DOI: 10.1111/bpa.12942
Cognitive decline in amyotrophic lateral sclerosis: Neuropathological substrate and genetic determinants
Abstract
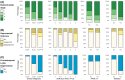
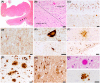
Cognitive impairment and behavioral changes in amyotrophic lateral sclerosis (ALS) are now recognized as part of the disease. Whether it is solely related to the extent of TDP-43 pathology is currently unclear. We aim to evaluate the influence of age, genetics, neuropathological features, and concomitant pathologies on cognitive impairment in ALS patients. We analyzed a postmortem series of 104 ALS patients and retrospectively reviewed clinical and neuropathological data. We assessed the burden and extent of concomitant pathologies, the role of APOE ε4 and mutations, and correlated these findings with cognitive status. We performed a logistic regression model to identify which pathologies are related to cognitive impairment. Cognitive decline was recorded in 38.5% of the subjects. Neuropathological features of frontotemporal lobar degeneration (FTLD) were found in 32.7%, explaining most, but not all, cases with cognitive impairment. Extent of TDP-43 pathology and the presence of hippocampal sclerosis were associated with cognitive impairment. Mutation carriers presented a higher burden of TDP-43 pathology and FTLD more frequently than sporadic cases. Most cases (89.4%) presented some degree of concomitant pathologies. The presence of concomitant pathologies was associated with older age at death. FTLD, but also Alzheimer's disease, were the predominant underlying pathologies explaining the cognitive impairment in ALS patients. In sum, FTLD explained the presence of cognitive decline in most but not all ALS cases, while other non-FTLD related findings can influence the cognitive status, particularly in older age groups.
Keywords: ALS-FTD; Alzheimer’s disease; TDP-43 protein; amyotrophic lateral sclerosis; frontotemporal dementia; neuropathology.
© 2021 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–80. - PubMed
-
- Prudlo J, König J, Schuster C, Kasper E, Büttner A, Teipel S, et al. TDP‐43 pathology and cognition in ALS. Neurology. 2016;87:1019–23. - PubMed
-
- Strong MJ, Grace GM, Freedman M, Lomen‐Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

