Vascular smooth muscle cells in atherosclerosis: time for a re-assessment
- PMID: 33576407
- PMCID: PMC8479803
- DOI: 10.1093/cvr/cvab046
Vascular smooth muscle cells in atherosclerosis: time for a re-assessment
Abstract
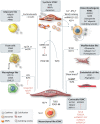
Vascular smooth muscle cells (VSMCs) are key participants in both early and late-stage atherosclerosis. VSMCs invade the early atherosclerotic lesion from the media, expanding lesions, but also forming a protective fibrous cap rich in extracellular matrix to cover the 'necrotic' core. Hence, VSMCs have been viewed as plaque-stabilizing, and decreased VSMC plaque content-often measured by expression of contractile markers-associated with increased plaque vulnerability. However, the emergence of lineage-tracing and transcriptomic studies has demonstrated that VSMCs comprise a much larger proportion of atherosclerotic plaques than originally thought, demonstrate multiple different phenotypes in vivo, and have roles that might be detrimental. VSMCs down-regulate contractile markers during atherosclerosis whilst adopting alternative phenotypes, including macrophage-like, foam cell-like, osteochondrogenic-like, myofibroblast-like, and mesenchymal stem cell-like. VSMC phenotypic switching can be studied in tissue culture, but also now in the media, fibrous cap and deep-core region, and markedly affects plaque formation and markers of stability. In this review, we describe the different VSMC plaque phenotypes and their presumed cellular and paracrine functions, the regulatory mechanisms that control VSMC plasticity, and their impact on atherogenesis and plaque stability.
Keywords: Atherosclerosis; Vascular smooth muscle.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R.. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13:79–98. - PubMed
-
- Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE.. Global overview of the epidemiology of atherosclerotic cardiovascular disease.Arch Med Res 2015;46:328–338. - PubMed
-
- Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P.. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res 2017;121:31–42. - PMC - PubMed
-
- Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R.. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. ATVB 2002;22:1642–1648. - PubMed
-
- Owens GK.Regulation of differentiation of vascular smooth muscle cells.Physiol Rev 1995;75:487–517. - PubMed
Publication types
MeSH terms
Grants and funding
- RG/08/009/25841/BHF_/British Heart Foundation/United Kingdom
- RG71070/BHF_/British Heart Foundation/United Kingdom
- CH/2000003/12800/BHF_/British Heart Foundation/United Kingdom
- RG84554/BHF_/British Heart Foundation/United Kingdom
- PG/16/24/32090/BHF_/British Heart Foundation/United Kingdom
- PG/16/63/32307/BHF_/British Heart Foundation/United Kingdom
- PG/16/11/32021/BHF_/British Heart Foundation/United Kingdom
- RG/13/14/30314/BHF_/British Heart Foundation/United Kingdom
- National Institute of Health Research Cambridge Biomedical Research Centre
- RG/20/2/34763/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

