Angiotensin II receptor 1 controls profibrotic Wnt/β-catenin signalling in experimental autoimmune myocarditis
- PMID: 33576779
- PMCID: PMC8803091
- DOI: 10.1093/cvr/cvab039
Angiotensin II receptor 1 controls profibrotic Wnt/β-catenin signalling in experimental autoimmune myocarditis
Abstract
Aims: Angiotensin (Ang) II signalling has been suggested to promote cardiac fibrosis in inflammatory heart diseases; however, the underlying mechanisms remain obscure. Using Agtr1a-/- mice with genetic deletion of angiotensin receptor type 1 (ATR1) and the experimental autoimmune myocarditis (EAM) model, we aimed to elucidate the role of Ang II-ATR1 pathway in development of heart-specific autoimmunity and post-inflammatory fibrosis.
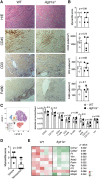
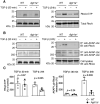
Methods and results: EAM was induced in wild-type (WT) and Agtr1a-/- mice by subcutaneous injections with alpha myosin heavy chain peptide emulsified in complete Freund's adjuvant. Agtr1a-/- mice developed myocarditis to a similar extent as WT controls at day 21 but showed reduced fibrosis and better systolic function at day 40. Crisscross bone marrow chimaera experiments proved that ATR1 signalling in the bone marrow compartment was critical for cardiac fibrosis. Heart infiltrating, bone-marrow-derived cells produced Ang II, but lack of ATR1 in these cells reduced transforming growth factor beta (TGF-β)-mediated fibrotic responses. At the molecular level, Agtr1a-/- heart-inflammatory cells showed impaired TGF-β-mediated phosphorylation of Smad2 and TAK1. In WT cells, TGF-β induced formation of RhoA-GTP and RhoA-A-kinase anchoring protein-Lbc (AKAP-Lbc) complex. In Agtr1a-/- cells, stabilization of RhoA-GTP and interaction of RhoA with AKAP-Lbc were largely impaired. Furthermore, in contrast to WT cells, Agtr1a-/- cells stimulated with TGF-β failed to activate canonical Wnt pathway indicated by suppressed activity of glycogen synthase kinase-3 (GSK-3)β and nuclear β-catenin translocation and showed reduced expression of Wnts. In line with these in vitro findings, β-catenin was detected in inflammatory regions of hearts of WT, but not Agtr1a-/- mice and expression of canonical Wnt1 and Wnt10b were lower in Agtr1a-/- hearts.
Conclusion: Ang II-ATR1 signalling is critical for development of post-inflammatory fibrotic remodelling and dilated cardiomyopathy. Our data underpin the importance of Ang II-ATR1 in effective TGF-β downstream signalling response including activation of profibrotic Wnt/β-catenin pathway.
Keywords: Angiotensin II; Angiotensin II receptor 1; Cardiac fibrosis; Experimental autoimmune myocarditis; Inflammatory cells; TGF-β signalling; Wnt.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures






Similar articles
-
TGF-β Signalling Regulates Cytokine Production in Inflammatory Cardiac Macrophages during Experimental Autoimmune Myocarditis.Int J Mol Sci. 2024 May 21;25(11):5579. doi: 10.3390/ijms25115579. Int J Mol Sci. 2024. PMID: 38891767 Free PMC article.
-
Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis.Eur Heart J. 2017 May 7;38(18):1413-1425. doi: 10.1093/eurheartj/ehw116. Eur Heart J. 2017. PMID: 27099262
-
Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis.Circ Res. 2009 Aug 28;105(5):462-70. doi: 10.1161/CIRCRESAHA.109.196287. Epub 2009 Jul 23. Circ Res. 2009. PMID: 19628793
-
Crosstalk between the TGF-β and WNT signalling pathways during cardiac fibrogenesis.Acta Biochim Pol. 2018;65(3):341-349. doi: 10.18388/abp.2018_2635. Epub 2018 Jul 24. Acta Biochim Pol. 2018. PMID: 30040870 Review.
-
Autoimmune myocarditis: cellular mediators of cardiac dysfunction.Autoimmun Rev. 2004 Nov;3(7-8):476-86. doi: 10.1016/j.autrev.2004.08.009. Autoimmun Rev. 2004. PMID: 15546794 Review.
Cited by
-
Cardiac Fibrosis and Fibroblasts.Cells. 2021 Jul 6;10(7):1716. doi: 10.3390/cells10071716. Cells. 2021. PMID: 34359886 Free PMC article. Review.
-
Complex regulation of cardiac fibrosis: insights from immune cells and signaling pathways.J Transl Med. 2025 Feb 28;23(1):242. doi: 10.1186/s12967-025-06260-5. J Transl Med. 2025. PMID: 40022104 Free PMC article. Review.
-
Comparison of COVID-19 Vaccine-Associated Myocarditis and Viral Myocarditis Pathology.Vaccines (Basel). 2023 Feb 5;11(2):362. doi: 10.3390/vaccines11020362. Vaccines (Basel). 2023. PMID: 36851240 Free PMC article. Review.
-
The role of WNT10B in physiology and disease: A 10-year update.Front Cell Dev Biol. 2023 Feb 6;11:1120365. doi: 10.3389/fcell.2023.1120365. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36814601 Free PMC article. Review.
-
TGF-β Signalling Regulates Cytokine Production in Inflammatory Cardiac Macrophages during Experimental Autoimmune Myocarditis.Int J Mol Sci. 2024 May 21;25(11):5579. doi: 10.3390/ijms25115579. Int J Mol Sci. 2024. PMID: 38891767 Free PMC article.
References
-
- Heymans S, Eriksson U, Lehtonen J, Cooper LT.. The quest for new approaches in myocarditis and inflammatory cardiomyopathy.J Am Coll Cardiol 2016;68:2348–2364. - PubMed
-
- Weintraub RG, Semsarian C, Macdonald P.. Dilated cardiomyopathy. The Lancet 2017;390:400–414. - PubMed
-
- Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H-P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM.. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. - PubMed
-
- Blyszczuk P, Berthonneche C, Behnke S, Glönkler M, Moch H, Pedrazzini T, Lüscher TF, Eriksson U, Kania G.. Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133+ progenitors into F4/80+ macrophages in experimental autoimmune myocarditis. Cardiovasc Res 2013;97:219–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

