American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease
- PMID: 33577086
- PMCID: PMC8014184
- DOI: 10.1002/hep.31751
American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease
Abstract
The aim of this document is to provide a concise scientific review of the currently available COVID-19 vaccines and those in development, including mRNA, adenoviral vectors, and recombinant protein approaches. The anticipated use of COVID-19 vaccines in patients with chronic liver disease (CLD) and liver transplant (LT) recipients is reviewed and practical guidance is provided for health care providers involved in the care of patients with liver disease and LT about vaccine prioritization and administration. The Pfizer and Moderna mRNA COVID-19 vaccines are associated with a 94%-95% vaccine efficacy compared to placebo against COVID-19. Local site reactions of pain and tenderness were reported in 70%-90% of clinical trial participants, and systemic reactions of fever and fatigue were reported in 40%-70% of participants, but these reactions were generally mild and self-limited and occurred more frequently in younger persons. Severe hypersensitivity reactions related to the mRNA COVID-19 vaccines are rare and more commonly observed in women and persons with a history of previous drug reactions for unclear reasons. Because patients with advanced liver disease and immunosuppressed patients were excluded from the vaccine licensing trials, additional data regarding the safety and efficacy of COVID-19 vaccines are eagerly awaited in these and other subgroups. Remarkably safe and highly effective mRNA COVID-19 vaccines are now available for widespread use and should be given to all adult patients with CLD and LT recipients. The online companion document located at https://www.aasld.org/about-aasld/covid-19-resources will be updated as additional data become available regarding the safety and efficacy of other COVID-19 vaccines in development.
© 2021 by the American Association for the Study of Liver Diseases.
Figures
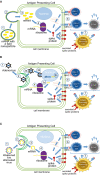
The mRNA is surrounded by a lipid nanoparticle.
The lipid nanoparticle assists with cell entry.
mRNA is released into the cytoplasm.
Ribosomes and cellular proteins are used to translate the mRNA into the spike protein.
The spike protein gets expressed on the cell surface and/or secreted into the serum.
The spike proteins expressed on the cell surface by the MHC receptors can activate T cells, which can activate the immune system, for additional T cells, B cells, and the production of antibodies against the spike protein.
Antigen‐presenting cells can engulf secreted spike proteins, which can also activate the immune system.
The adenovirus contains DNA, which includes genetic material to produce the spike protein.
The adenovirus is taken up by the human cell.
The adenovirus enters the cytoplasm.
The adenovirus releases its DNA into the nucleus.
Transcription of the DNA to mRNA occurs in the nucleus.
mRNA is transferred into the cytoplasm.
Ribosomes and cellular proteins are used to translate the mRNA into the spike protein.
The spike protein gets expressed on the cell surface and/or secreted into the serum.
The spike proteins expressed on the cell surface by the MHC receptors can activate T cells, which can activate the immune system, for additional T cells, B cells, and the production of antibodies against the spike protein.
Antigen‐presenting cells can engulf secreted spiked proteins, which can also activate the immune system.
Weakened live attenuated virus containing the mRNA of the spike protein
The attenuated virus binds to the ACE2 for cell entry.
mRNA is released into the cytoplasm.
Ribosomes and cellular proteins are used to translate the mRNA into the spiked protein.
The spike protein gets expressed on the cell surface and/or secreted into the serum.
The spike proteins expressed on the cell surface by the MHC receptors can activate T cells, which can activate the immune system, for additional T cells, B cells, and the production of antibodies against the spike protein.
Antigen‐presenting cells can engulf secreted spiked proteins, which can also activate the immune system.
References
-
- Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:727‐738. - PubMed
-
- van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, George R, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012;65:17‐24. - PubMed
-
- Gutierrez Domingo I, Pascasio Acevedo JM, Alcalde Vargas A, Ramos Cuadra A, Ferrer Ríos MT, Sousa Martin JM, et al. Response to vaccination against hepatitis B virus with a schedule of four 40‐μg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc 2012;44:1499‐1501. - PubMed
-
- Bonazzi PR, Bacchella T, Freitas AC, Osaki KT, Lopes MH, Freire MP, et al. Double‐dose hepatitis B vaccination in cirrhotic patients on a liver transplant waiting list. Braz J Infect Dis 2008;12:306‐309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

