Air Hunger: A Primal Sensation and a Primary Element of Dyspnea
- PMID: 33577128
- PMCID: PMC10986303
- DOI: 10.1002/cphy.c200001
Air Hunger: A Primal Sensation and a Primary Element of Dyspnea
Abstract
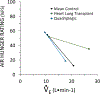
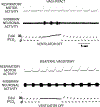
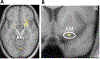
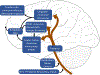
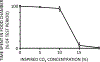
The sensation that develops as a long breath hold continues is what this article is about. We term this sensation of an urge to breathe "air hunger." Air hunger, a primal sensation, alerts us to a failure to meet an urgent homeostatic need maintaining gas exchange. Anxiety, frustration, and fear evoked by air hunger motivate behavioral actions to address the failure. The unpleasantness and emotional consequences of air hunger make it the most debilitating component of clinical dyspnea, a symptom associated with respiratory, cardiovascular, and metabolic diseases. In most clinical populations studied, air hunger is the predominant form of dyspnea (colloquially, shortness of breath). Most experimental subjects can reliably quantify air hunger using rating scales, that is, there is a consistent relationship between stimulus and rating. Stimuli that increase air hunger include hypercapnia, hypoxia, exercise, and acidosis; tidal expansion of the lungs reduces air hunger. Thus, the defining experimental paradigm to evoke air hunger is to elevate the drive to breathe while mechanically restricting ventilation. Functional brain imaging studies have shown that air hunger activates the insular cortex (an integration center for perceptions related to homeostasis, including pain, food hunger, and thirst), as well as limbic structures involved with anxiety and fear. Although much has been learned about air hunger in the past few decades, much remains to be discovered, such as an accepted method to quantify air hunger in nonhuman animals, fundamental questions about neural mechanisms, and adequate and safe methods to mitigate air hunger in clinical situations. © 2021 American Physiological Society. Compr Physiol 11:1449-1483, 2021.
Copyright © 2021 American Physiological Society. All rights reserved.
Figures


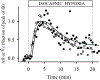


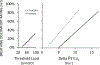
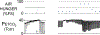
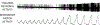


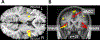
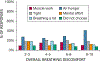
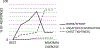
References
-
- Adams L, Lane R, Shea SA, Cockcroft A, and Guz A. Breathlessness during different forms of ventilatory stimulation: a study of mechanisms in normal subjects and respiratory patients. Clin Sci 69: 663–672, 1985. - PubMed
-
- Amour FE, and Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther 72: 74, 1941.
-
- Anton F, Euchner I, and Handwerker HO. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 49: 53–60, 1992. - PubMed
-
- Anton F, Peppel P, Euchner I, and Handwerker HO. Controlled noxious chemical stimulation: responses of rat trigeminal brainstem neurones to CO2 pulses applied to the nasal mucosa. Neurosci Lett 123: 208–211, 1991. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

