Evaluation of an Interprofessional Training Program to Improve Cancer Drug Therapy Safety
- PMID: 33577351
- PMCID: PMC9810130
- DOI: 10.1200/OP.20.00816
Evaluation of an Interprofessional Training Program to Improve Cancer Drug Therapy Safety
Abstract
Purpose: Drug therapy for cancer is a high-risk, high-volume clinical intervention that requires interprofessional teams. Given the complexity of anticancer drug therapy and safety concerns, an interdisciplinary team developed a novel training program for oncology registered nurses and pharmacists to improve cancer drug safety.
Methods: Participants completed preworkshop learning assessments and received access to web-based modules on six topics: hazardous drug handling, drug extravasation, hypersensitivity reaction management, sepsis recognition, immune checkpoint inhibitor toxicities, and oral oncolytic adherence. In a 7-hour workshop, participants applied module content in interactive exercises and high-fidelity simulations. Preworkshop and postworkshop questionnaires assessed changes in knowledge and confidence in each topic. Program satisfaction and changes to clinical practice or policies were assessed 3 months after the workshop.
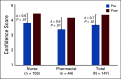
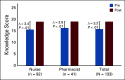
Results: Two hundred ninety-two nurses and 82 pharmacists applied to participate, and 103 (35%) and 44 (54%) have participated, respectively. Long-term follow-up data were available on 133 (90%) participants. Change scores in confidence to meet program objectives increased between pre- and postworkshop (range of increase 0.6-0.8, P < .01). Knowledge scores increased significantly between pre- and postworkshop (average improvement of 3.2 points, P < .01). Overall program satisfaction was high (mean 5.0, standard deviation [0.2] on a five-point scale). Seventy-seven (60%) reported that they had made at least one clinical practice or institutional policy change at 3 months.
Conclusion: An interprofessional education program with online modules, in-person interactive sessions, and simulation activities is a promising strategy to deliver cancer drug safety content to practicing oncology clinicians.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention, National Center for Health Statistics : Ambulatory health care data. http://www.cdc.gov/nchs/ahcd.htm
-
- Neuss MN, Polovich M, McNiff K, et al. : 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. Oncol Nurs Forum 40:225-233, 2013 - PubMed
-
- Jacobson JO, Polovich M, McNiff KK, et al. : American Society of Clinical oncology/Oncology Nursing Society chemotherapy administration safety standards. Oncol Nurs Forum 36:651-658, 2009 - PubMed
-
- Hematology/Oncology Pharmacy Association : Further Defining the Scope of Hematology/Oncology Pharmacy Practice. Chicago, IL, Hematology/Oncology Pharmacy Association, 2019
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

