Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma
- PMID: 33577729
- PMCID: PMC8450892
- DOI: 10.1056/NEJMoa2035807
Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma
Abstract
Background: Patients with advanced urothelial carcinoma have poor overall survival after platinum-containing chemotherapy and programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitor treatment.
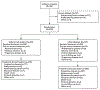
Methods: We conducted a global, open-label, phase 3 trial of enfortumab vedotin for the treatment of patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-containing chemotherapy and had had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. Patients were randomly assigned in a 1:1 ratio to receive enfortumab vedotin (at a dose of 1.25 mg per kilogram of body weight on days 1, 8, and 15 of a 28-day cycle) or investigator-chosen chemotherapy (standard docetaxel, paclitaxel, or vinflunine), administered on day 1 of a 21-day cycle. The primary end point was overall survival.
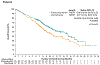
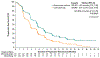
Results: A total of 608 patients underwent randomization; 301 were assigned to receive enfortumab vedotin and 307 to receive chemotherapy. As of July 15, 2020, a total of 301 deaths had occurred (134 in the enfortumab vedotin group and 167 in the chemotherapy group). At the prespecified interim analysis, the median follow-up was 11.1 months. Overall survival was longer in the enfortumab vedotin group than in the chemotherapy group (median overall survival, 12.88 vs. 8.97 months; hazard ratio for death, 0.70; 95% confidence interval [CI], 0.56 to 0.89; P = 0.001). Progression-free survival was also longer in the enfortumab vedotin group than in the chemotherapy group (median progression-free survival, 5.55 vs. 3.71 months; hazard ratio for progression or death, 0.62; 95% CI, 0.51 to 0.75; P<0.001). The incidence of treatment-related adverse events was similar in the two groups (93.9% in the enfortumab vedotin group and 91.8% in the chemotherapy group); the incidence of events of grade 3 or higher was also similar in the two groups (51.4% and 49.8%, respectively).
Conclusions: Enfortumab vedotin significantly prolonged survival as compared with standard chemotherapy in patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-based treatment and a PD-1 or PD-L1 inhibitor. (Funded by Astellas Pharma US and Seagen; EV-301 ClinicalTrials.gov number, NCT03474107.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
Benefit with enfortumab vedotin confirmed.Nat Rev Clin Oncol. 2021 May;18(5):258. doi: 10.1038/s41571-021-00493-1. Nat Rev Clin Oncol. 2021. PMID: 33649567 No abstract available.
-
Benefit of nectin-4 targeting with enfortumab vedotin confirmed.Nat Rev Urol. 2021 Apr;18(4):190. doi: 10.1038/s41585-021-00449-1. Nat Rev Urol. 2021. PMID: 33692500 No abstract available.
-
Re: Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma.Eur Urol. 2021 Aug;80(2):257-258. doi: 10.1016/j.eururo.2021.04.007. Epub 2021 Apr 22. Eur Urol. 2021. PMID: 33895011 No abstract available.
-
Re: Thomas Powles, Jonathan E. Rosenberg, Guru P. Sonpavde, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021;384:1125-35.Eur Urol Oncol. 2021 Aug;4(4):670. doi: 10.1016/j.euo.2021.04.010. Epub 2021 May 13. Eur Urol Oncol. 2021. PMID: 33994337 No abstract available.
-
Enfortumab Vedotin in Advanced Urothelial Carcinoma.N Engl J Med. 2021 Jul 1;385(1):93. doi: 10.1056/NEJMc2106975. N Engl J Med. 2021. PMID: 34192440 No abstract available.
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2022 Jan;207(1):228-229. doi: 10.1097/JU.0000000000002268. Epub 2021 Oct 18. J Urol. 2022. PMID: 34661437 No abstract available.
References
-
- Bellmunt J, Orsola A, Leow JJ, et al.Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25Suppl 3:iii40–8. - PubMed
-
- Bladder Cancer (Version 6.2020). National Comprehensive Cancer Network. (Accessed October 16, 2020, at https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.o....)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
