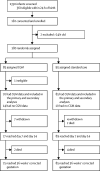
Real-time continuous glucose monitoring in preterm infants (REACT): an international, open-label, randomised controlled trial
- PMID: 33577770
- PMCID: PMC7970623
- DOI: 10.1016/S2352-4642(20)30367-9
Real-time continuous glucose monitoring in preterm infants (REACT): an international, open-label, randomised controlled trial
Abstract
Background: Hyperglycaemia and hypoglycaemia are common in preterm infants and have been associated with increased risk of mortality and morbidity. Interventions to reduce risk associated with these exposures are particularly challenging due to the infrequent measurement of blood glucose concentrations, with the potential of causing more harm instead of improving outcomes for these infants. Continuous glucose monitoring (CGM) is widely used in adults and children with diabetes to improve glucose control, but has not been approved for use in neonates. The REACT trial aimed to evaluate the efficacy and safety of CGM in preterm infants requiring intensive care.
Methods: This international, open-label, randomised controlled trial was done in 13 neonatal intensive care units in the UK, Spain, and the Netherlands. Infants were included if they were within 24 h of birth, had a birthweight of 1200 g or less, had a gestational age up to 33 weeks plus 6 days, and had parental written informed consent. Infants were randomly assigned (1:1) to real-time CGM or standard care (with masked CGM for comparison) using a central web randomisation system, stratified by recruiting centre and gestational age (<26 or ≥26 weeks). The primary efficacy outcome was the proportion of time sensor glucose concentration was 2·6-10 mmol/L for the first week of life. Safety outcomes related to hypoglycaemia (glucose concentrations <2·6 mmol/L) in the first 7 days of life. All outcomes were assessed on the basis of intention to treat in the full analysis set with available data. The study is registered with the International Standard Randomised Control Trials Registry, ISRCTN12793535.
Findings: Between July 4, 2016, and Jan 27, 2019, 182 infants were enrolled, 180 of whom were randomly assigned (85 to real-time CGM, 95 to standard care). 70 infants in the real-time CGM intervention group and 85 in the standard care group had CGM data and were included in the primary analysis. Compared with infants in the standard care group, infants managed using CGM had more time in the 2·6-10 mmol/L glucose concentration target range (mean proportion of time 84% [SD 22] vs 94% [11]; adjusted mean difference 8·9% [95% CI 3·4-14·4]), equivalent to 13 h (95% CI 5-21). More infants in the standard care group were exposed to at least one episode of sensor glucose concentration of less than 2·6 mmol/L for more than 1 h than those in the intervention group (13 [15%] of 85 vs four [6%] of 70). There were no serious adverse events related to the use of the device or episodes of infection.
Interpretation: Real-time CGM can reduce exposure to prolonged or severe hyperglycaemia and hypoglycaemia. Further studies using CGM are required to determine optimal glucose targets, strategies to obtain them, and the potential effect on long-term health outcomes.
Funding: National Institute for Health Research Efficacy and Mechanisms Evaluation Programme.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
New perspectives on real-time continuous glucose monitoring.Lancet Child Adolesc Health. 2021 Apr;5(4):235-236. doi: 10.1016/S2352-4642(20)30393-X. Epub 2021 Feb 10. Lancet Child Adolesc Health. 2021. PMID: 33577769 No abstract available.
-
Continuous glucose monitoring in the neonatal intensive care unit: need for practical guidelines.Lancet Child Adolesc Health. 2021 May;5(5):e15. doi: 10.1016/S2352-4642(21)00096-1. Lancet Child Adolesc Health. 2021. PMID: 33864743 No abstract available.
-
Continuous glucose monitoring in the neonatal intensive care unit: need for practical guidelines - Authors' reply.Lancet Child Adolesc Health. 2021 May;5(5):e16. doi: 10.1016/S2352-4642(21)00093-6. Lancet Child Adolesc Health. 2021. PMID: 33864744 No abstract available.
-
Real-time continuous glucose monitoring in preterm infants (react): An International, open-label, randomised, controlled trial.Acta Paediatr. 2021 Sep;110(9):2656-2657. doi: 10.1111/apa.15946. Epub 2021 Jun 13. Acta Paediatr. 2021. PMID: 34121224 No abstract available.
References
-
- Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23:186–194. - PubMed
-
- Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26:730–736. - PubMed
-
- Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118:1811–1818. - PubMed
-
- Yager JY. Hypoglycemic injury to the immature brain. Clin Perinatol. 2002;29:651–674. - PubMed
-
- Finberg L. Dangers to infants caused by changes in osmolal concentration. Pediatrics. 1967;40:1031–1034. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical