RETRACTED: Human DNA polymerase θ harbors DNA end-trimming activity critical for DNA repair
- PMID: 33577776
- PMCID: PMC8231307
- DOI: 10.1016/j.molcel.2021.01.021
RETRACTED: Human DNA polymerase θ harbors DNA end-trimming activity critical for DNA repair
Retraction in
-
Retraction Notice to: Human DNA polymerase θ harbors DNA end-trimming activity critical for DNA repair.Mol Cell. 2024 Apr 18;84(8):1626. doi: 10.1016/j.molcel.2024.03.024. Mol Cell. 2024. PMID: 38640897 Free PMC article. No abstract available.
Abstract
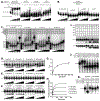
Cancers with hereditary defects in homologous recombination rely on DNA polymerase θ (pol θ) for repair of DNA double-strand breaks. During end joining, pol θ aligns microhomology tracts internal to 5'-resected broken ends. An unidentified nuclease trims the 3' ends before synthesis can occur. Here we report that a nuclease activity, which differs from the proofreading activity often associated with DNA polymerases, is intrinsic to the polymerase domain of pol θ. Like the DNA synthesis activity, the nuclease activity requires conserved metal-binding residues, metal ions, and dNTPs and is inhibited by ddNTPs or chain-terminated DNA. Our data indicate that pol θ repurposes metal ions in the polymerase active site for endonucleolytic cleavage and that the polymerase-active and end-trimming conformations of the enzyme are distinct. We reveal a nimble strategy of substrate processing that allows pol θ to trim or extend DNA depending on the DNA repair context.
Keywords: DNA polymerase; Double-strand break repair; dideoxynucleotides; endonuclease; theta-mediated end joining (TMEJ).
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.D.W. is a scientific advisor for Repare Therapeutics and a shareholder. R.B.J. is named on patent US9150897B2, which references the phCMV1-2xMBP vector adapted by this study to express pol θ-FL.
Figures
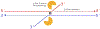




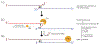
References
-
- Astatke M, Grindley ND, and Joyce CM (1995). Deoxynucleoside triphosphate and pyrophosphate binding sites in the catalytically competent ternary complex for the polymerase reaction catalyzed by DNA polymerase I (Klenow fragment). J Biol Chem 270, 1945–1954. - PubMed
-
- Astatke M, Grindley ND, and Joyce CM (1998). How E. coli DNA polymerase I (Klenow fragment) distinguishes between deoxy- and dideoxynucleotides. J Mol Biol 278, 147–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

