Rationale and design for the study of rivaroxaban to reduce thrombotic events, hospitalization and death in outpatients with COVID-19: The PREVENT-HD study
- PMID: 33577800
- PMCID: PMC7871775
- DOI: 10.1016/j.ahj.2021.02.001
Rationale and design for the study of rivaroxaban to reduce thrombotic events, hospitalization and death in outpatients with COVID-19: The PREVENT-HD study
Abstract
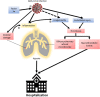
Background: COVID-19 is associated with both venous and arterial thrombotic complications. While prophylactic anticoagulation is now widely recommended for hospitalized patients with COVID-19, the effectiveness and safety of thromboprophylaxis in outpatients with COVID-19 has not been established.
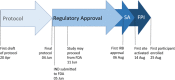
Study design: PREVENT-HD is a double-blind, placebo-controlled, pragmatic, event-driven phase 3 trial to evaluate the efficacy and safety of rivaroxaban in symptomatic outpatients with laboratory-confirmed COVID-19 at risk for thrombotic events, hospitalization, and death. Several challenges posed by the pandemic have necessitated innovative approaches to clinical trial design, start-up, and conduct. Participants are randomized in a 1:1 ratio, stratified by time from COVID-19 confirmation, to either rivaroxaban 10 mg once daily or placebo for 35 days. The primary efficacy end point is a composite of symptomatic venous thromboembolism, myocardial infarction, ischemic stroke, acute limb ischemia, non-central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality. The primary safety end point is fatal and critical site bleeding according to the International Society on Thrombosis and Haemostasis definition. Enrollment began in August 2020 and is expected to enroll approximately 4,000 participants to yield the required number of end point events.
Conclusions: PREVENT-HD is a pragmatic trial evaluating the efficacy and safety of the direct oral anticoagulant rivaroxaban in the outpatient setting to reduce major venous and arterial thrombotic events, hospitalization, and mortality associated with COVID-19.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


References
-
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

