Quantitative CT metrics are associated with longitudinal lung function decline and future asthma exacerbations: Results from SARP-3
- PMID: 33577895
- PMCID: PMC8349941
- DOI: 10.1016/j.jaci.2021.01.029
Quantitative CT metrics are associated with longitudinal lung function decline and future asthma exacerbations: Results from SARP-3
Abstract
Background: Currently, there is limited knowledge regarding which imaging assessments of asthma are associated with accelerated longitudinal decline in lung function.
Objectives: We aimed to assess whether quantitative computed tomography (qCT) metrics are associated with longitudinal decline in lung function and morbidity in asthma.
Methods: We analyzed 205 qCT scans of adult patients with asthma and calculated baseline markers of airway remodeling, lung density, and pointwise regional change in lung volume (Jacobian measures) for each participant. Using multivariable regression models, we then assessed the association of qCT measurements with the outcomes of future change in lung function, future exacerbation rate, and changes in validated measurements of morbidity.
Results: Greater baseline wall area percent (β = -0.15 [95% CI = -0.26 to -0.05]; P < .01), hyperinflation percent (β = -0.25 [95% CI = -0.41 to -0.09]; P < .01), and Jacobian gradient measurements (cranial-caudal β = 10.64 [95% CI = 3.79-17.49]; P < .01; posterior-anterior β = -9.14, [95% CI = -15.49 to -2.78]; P < .01) were associated with more severe future lung function decline. Additionally, greater wall area percent (rate ratio = 1.06 [95% CI = 1.01-1.10]; P = .02) and air trapping percent (rate ratio =1.01 [95% CI = 1.00-1.02]; P = .03), as well as lower decline in the Jacobian determinant mean (rate ratio = 0.58 [95% CI = 0.41-0.82]; P < .01) and Jacobian determinant standard deviation (rate ratio = 0.52 [95% CI = 0.32-0.85]; P = .01), were associated with a greater rate of future exacerbations. However, imaging metrics were not associated with clinically meaningful changes in scores on validated asthma morbidity questionnaires.
Conclusions: Baseline qCT measures of more severe airway remodeling, more small airway disease and hyperinflation, and less pointwise regional change in lung volumes were associated with future lung function decline and asthma exacerbations.
Keywords: Asthma; CT imaging; asthma exacerbations; asthma morbidity; longitudinal; lung function; severe asthma.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
J.K., C.W.G., D.L., M.S., M.C.M., J.B., A.S., C.H., J.B., K.B.S., S.P., and S.M. have no conflicts of interest. L.B.B. reports grants from the NIH/NHLBI, during the conduct of the study; personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees and non-financial support from Merck, personal fees from DBV Technologies, personal fees and non-financial support from Teva, personal fees and non-financial support from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from WebMD/Medscape, personal fees from Sanofi/Regeneron, personal fees from Vectura, personal fees from Circassia, outside the submitted work. D.T.M. reports grants from the NIH, grants from Boehringer-Ingelheim, grants from TEVA, grants from AstraZeneca, grants from GlaxoSmithKline, grants from Sanofi, grants from Genentech, during the conduct of the study; non-financial support from Vifor-Pharma, non-financial support from Merck, outside the submitted work. J.V.H. reports grants from NIH/NHLBI, grants from Boehringer Ingelheim, during the conduct of the study; personal fees from Boehringer Ingelheim, personal fees from Pieris, personal fees from Arrowhead Pharmaceuticals, personal fees from Gossamer, outside the submitted work; In addition, Dr. Fahy has a patent US20110123530A1 - “Compositions and methods for treating and diagnosing asthma” issued, a patent WO2014153009A2 - Thiosaccharide mucolytic agents issued, and a patent WO2017197360 - “CT Mucus Score” - A new scoring system that quantifies airway mucus impaction using CT lung scans. S.B.F. reports grants from NIH/NHLBI, during the conduct of the study; personal fees from COPD Gene Foundation, personal fees from Sanofi/Regeneron, grants from GE Healthcare, outside the submitted work; and Serves as the Physics Chair of the CT lung density biomarker committee within the Quantitative Imaging Biomarker Alliance (QIBA). L.C.D. reports grants from NHLBI, during the conduct of the study; grants and personal fees from AstraZeneca, personal fees from Sanofi, grants from TEVA, outside the submitted work. G.W. reports grants from NIH, grants and other from Boehringer Ingelheim, other from Quantitative Imaging Solutions, other from PulmonX, grants from BTG Interventional Medicine, grants and other from Janssen Pharmaceuticals, other from GlaxoSmithKline, other from Novartis, other from Vertex, outside the submitted work; and G.W.’s spouse works for Biogen. E.I. reports grants from AstraZeneca, non-financial support from GlaxoSmithKline, during the conduct of the study; personal fees from AB Science, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Avillion, personal fees from Biometry, personal fees from Equillium, personal fees from Merck, grants and personal fees from Novartis, personal fees from 4D Pharma, personal fees from Pneuma Respiratory, personal fees from PPS Health, personal fees from Regeneron, personal fees from Sanofi Genzyme, personal fees from Sienna Biopharmaceutical, other from Vorso Corp, grants, personal fees and non-financial support from Genentech, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from TEVA, grants from Gossamer Bio, grants and non-financial support from Circassia, non-financial support from Boehringer Ingelheim, outside the submitted work; E.H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. S.E.W. reports grants from Boerhringer-Ingelheim to support SARP3 visits, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from GSK, grants and personal fees from Sanofi, grants from Novartis, personal fees from Pieris, outside the submitted work. M.C. receives University Grant Funding from NIH, American Lung Association, PCORI; receives Pharmaceutical Grant Funding from AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, Shionogi; is a consultant for Genentech, Teva, Sanofi-Aventis, Novartis; he is a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva; and receives Royalties from Elsevier.
Figures
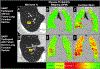
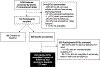

Comment in
-
Airway remodeling: Shifting the trigger point for exacerbations in asthma.J Allergy Clin Immunol. 2021 Sep;148(3):710-712. doi: 10.1016/j.jaci.2021.07.010. Epub 2021 Jul 23. J Allergy Clin Immunol. 2021. PMID: 34310927 No abstract available.
References
-
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–78. - PubMed
-
- Matsunaga K, Akamatsu K, Miyatake A, Ichinose M. Natural history and risk factors of obstructive changes over a 10-year period in severe asthma. Respir Med 2013; 107:355–60. - PubMed
-
- ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med 2001; 164:744–8. - PubMed
-
- Covar RA, Spahn JD, Murphy JR, Szefler SJ, Childhood Asthma Management Program Research G. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med 2004; 170:234–41. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR000427/TR/NCATS NIH HHS/United States
- U10 HL109172/HL/NHLBI NIH HHS/United States
- R01 HL091762/HL/NHLBI NIH HHS/United States
- U10 HL109257/HL/NHLBI NIH HHS/United States
- U01 HL146002/HL/NHLBI NIH HHS/United States
- R01 HL069149/HL/NHLBI NIH HHS/United States
- T32 HL007317/HL/NHLBI NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- U10 HL109086/HL/NHLBI NIH HHS/United States
- S10 OD025214/OD/NIH HHS/United States
- U10 HL109168/HL/NHLBI NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- U10 HL109152/HL/NHLBI NIH HHS/United States
- U10 HL109146/HL/NHLBI NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States

