Analysis of 17,428 pregnant women undergoing non-invasive prenatal testing for fetal chromosome in Northeast China
- PMID: 33578623
- PMCID: PMC10545248
- DOI: 10.1097/MD.0000000000024740
Analysis of 17,428 pregnant women undergoing non-invasive prenatal testing for fetal chromosome in Northeast China
Abstract
Non-invasive prenatal testing (NIPT) is an incomparable prenatal screening technology, but we should undergo amniocentesis to confirm fetal chromosome when pregnancies receive a positive result via NIPT. We aimed to investigate the detection rate and positive predictive value of NIPT results in pregnancies from Northeast China, and to determine the reasons for false positive and false negative NIPT results.This study evaluates 17,428 singleton pregnancies had undergone NIPT detection. 202 samples were NIPT positive with the detection rate was 1.16% (202/17,428). Among all the positive samples, 160 samples (79.21%) were referred for an amniocentesis procedure to investigate the fetal chromosome. The positive predictive value of T21, T18, and T13 was found to be 75% with a 0.07% false positive rate. Positive predictive value from high to low was as follows: trisomy 21 (84.38%), followed by trisomy 18 (61.54%), autosomal abnormalities (52.94%), sex chromosomal abnormalities (38.46%), and trisomy 13 (33.33%). The positive predictive values for sex chromosome abnormalities turned out to be mosaic sex chromosome aneuploidies (83.33%), followed by XYY (57.14%), XXY (37.50%), XXX (36.36%), and Monosomy X (28.95%). Out of the 160 samples had amniocentesis, the true positive cases in trisomy 21 had a higher percentage of Z-scores compared with the false positive cases in trisomy 21 (P < .05). And the true positive cases in trisomy 18 had a significantly higher percentage of Z-scores compared with the false positive cases in trisomy 18 (P < .01).These findings indicate that the positive predictive value of T21, T18, and T13 was found to be 75% with a 0.07% false positive rate. It is worth noting that the positive predictive value of NIPT for autosomes and sex chromosomes. Moreover, if women receive a positive result via NIPT, they should pay attention to the results with undergoing further prenatal diagnosis.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures
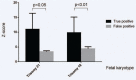
References
-
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485–7. - PubMed
-
- Hartwig TS, Ambye L, Sørensen S, et al. Discordant non-invasive prenatal testing (NIPT) - a systematic review. Prenat Diagn 2017;37:527–39. - PubMed
-
- Benn P, Borrell A, Chiu RW, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn 2015;35:725–34. - PubMed
-
- Di Renzo GC, Bartha JL, Bilardo CM. Expanding the indications for cell-free DNA in the maternal circulation: clinical considerations and implications. Am J Obstet Gynecol 2019;220:537–42. - PubMed
-
- Shaw J, Scotchman E, Chandler N, et al. Non-invasive prenatal testing for aneuploidy, copy number variants and single gene disorders. Reproduction 2020;1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

