In Vitro and In Vivo Anti- Candida Activity and Structural Analysis of Killer Peptide (KP)-Derivatives
- PMID: 33578728
- PMCID: PMC7916522
- DOI: 10.3390/jof7020129
In Vitro and In Vivo Anti- Candida Activity and Structural Analysis of Killer Peptide (KP)-Derivatives
Abstract
The previously described decapeptide AKVTMTCSAS (killer peptide, KP), derived from the variable region of a recombinant yeast killer toxin-like anti-idiotypic antibody, proved to exert a variety of antimicrobial, antiviral, and immunomodulatory activities. It also showed a peculiar self-assembly ability, likely responsible for the therapeutic effect in animal models of systemic and mucosal candidiasis. The present study analyzed the biological and structural properties of peptides derived from KP by substitution or deletion of the first residue, leaving unchanged the remaining amino acids. The investigated peptides proved to exert differential in vitro and/or in vivo anti-Candida activity without showing toxic effects on mammalian cells. The change of the first residue in KP amino acidic sequence affected the conformation of the resulting peptides in solution, as assessed by circular dichroism spectroscopy. KP-derivatives, except one, were able to induce apoptosis in yeast cells, like KP itself. ROS production and changes in mitochondrial transmembrane potential were also observed. Confocal and transmission electron microscopy studies allowed to establish that selected peptides could penetrate within C. albicans cells and cause gross morphological alterations. Overall, the physical and chemical properties of the first residue were found to be important for peptide conformation, candidacidal activity and possible mechanism of action. Small antimicrobial peptides could be exploited for the development of a new generation of antifungal drugs, given their relative low cost and ease of production as well as the possibility of devising novel delivery systems.
Keywords: Candida albicans; Galleria mellonella model; antifungal peptides; circular dichroism spectroscopy; confocal microscopy; electron microscopy; self-assembly peptides; structure-function relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
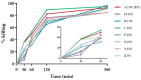
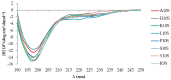


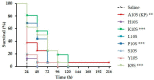









References
-
- World Health Organization . Global Action Plan on Antimicrobial Resistance. WHO; Geneva, Switzerland: 2015. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

