Central Role of Dendritic Cells in Pulmonary Arterial Hypertension in Human and Mice
- PMID: 33578743
- PMCID: PMC7916474
- DOI: 10.3390/ijms22041756
Central Role of Dendritic Cells in Pulmonary Arterial Hypertension in Human and Mice
Abstract
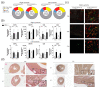
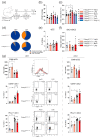
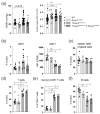
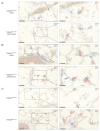
The pathogenesis of idiopathic pulmonary arterial hypertension (IPAH) is not fully understood, but evidence is accumulating that immune dysfunction plays a significant role. We previously reported that 31-week-old Tnfaip3DNGR1-KO mice develop pulmonary hypertension (PH) symptoms. These mice harbor a targeted deletion of the TNFα-induced protein-3 (Tnfaip3) gene, encoding the NF-κB regulatory protein A20, specifically in type I conventional dendritic cells (cDC1s). Here, we studied the involvement of dendritic cells (DCs) in PH in more detail. We found various immune cells, including DCs, in the hearts of Tnfaip3DNGR1-KO mice, particularly in the right ventricle (RV). Secondly, in young Tnfaip3DNGR1-KO mice, innate immune activation through airway exposure to toll-like receptor ligands essentially did not result in elevated RV pressures, although we did observe significant RV hypertrophy. Thirdly, PH symptoms in Tnfaip3DNGR1-KO mice were not enhanced by concomitant mutation of bone morphogenetic protein receptor type 2 (Bmpr2), which is the most affected gene in PAH patients. Finally, in human IPAH lung tissue we found co-localization of DCs and CD8+ T cells, representing the main cell type activated by cDC1s. Taken together, these findings support a unique role of cDC1s in PAH pathogenesis, independent of general immune activation or a mutation in the Bmpr2 gene.
Keywords: BMPR2; Tnfaip3; Toll-like receptor; dendritic cells; inflammation; pulmonary arterial hypertension.
Conflict of interest statement
D.M. reports grants of and personal fees from Actelion, grants and personal fees from Bayer, personal fees from GSK, personal fees from Pfizer, grants, personal fees and non-financial support from MSD, personal fees from Chiesi, personal fees from Boerhinger, non-financial support from Acceleron, personal fees from Incyte Biosciences France, outside the submitted work. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures

Similar articles
-
DNGR1-Cre-mediated Deletion of Tnfaip3/A20 in Conventional Dendritic Cells Induces Pulmonary Hypertension in Mice.Am J Respir Cell Mol Biol. 2020 Nov;63(5):665-680. doi: 10.1165/rcmb.2019-0443OC. Am J Respir Cell Mol Biol. 2020. PMID: 32755457
-
DNGR1-mediated deletion of A20/Tnfaip3 in dendritic cells alters T and B-cell homeostasis and promotes autoimmune liver pathology.J Autoimmun. 2019 Aug;102:167-178. doi: 10.1016/j.jaut.2019.05.007. Epub 2019 Jul 9. J Autoimmun. 2019. PMID: 31151831
-
Tnfaip3 expression in pulmonary conventional type 1 Langerin-expressing dendritic cells regulates T helper 2-mediated airway inflammation in mice.Allergy. 2020 Oct;75(10):2587-2598. doi: 10.1111/all.14334. Epub 2020 Jun 14. Allergy. 2020. PMID: 32329078 Free PMC article.
-
Dendritic Cell Subsets and Effector Function in Idiopathic and Connective Tissue Disease-Associated Pulmonary Arterial Hypertension.Front Immunol. 2019 Jan 22;10:11. doi: 10.3389/fimmu.2019.00011. eCollection 2019. Front Immunol. 2019. PMID: 30723471 Free PMC article. Review.
-
A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models.Front Immunol. 2018 Feb 21;9:104. doi: 10.3389/fimmu.2018.00104. eCollection 2018. Front Immunol. 2018. PMID: 29515565 Free PMC article. Review.
Cited by
-
RPL39 Was Associated With Sex Differences in Pulmonary Arterial Hypertension.Can Respir J. 2025 Jan 28;2025:7139235. doi: 10.1155/carj/7139235. eCollection 2025. Can Respir J. 2025. PMID: 39957991 Free PMC article.
-
Classical dendritic cells contribute to hypoxia-induced pulmonary hypertension.FASEB J. 2024 Aug 31;38(16):e70015. doi: 10.1096/fj.202400338RR. FASEB J. 2024. PMID: 39212294
-
Therapeutic Approaches for Treating Pulmonary Arterial Hypertension by Correcting Imbalanced TGF-β Superfamily Signaling.Front Med (Lausanne). 2022 Jan 24;8:814222. doi: 10.3389/fmed.2021.814222. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35141256 Free PMC article. Review.
-
Smouldering fire or conflagration? An illustrated update on the concept of inflammation in pulmonary arterial hypertension.Eur Respir Rev. 2021 Dec 22;30(162):210161. doi: 10.1183/16000617.0161-2021. Print 2021 Dec 31. Eur Respir Rev. 2021. PMID: 34937704 Free PMC article. Review.
-
Role of macrophages in pulmonary arterial hypertension.Front Immunol. 2023 Apr 19;14:1152881. doi: 10.3389/fimmu.2023.1152881. eCollection 2023. Front Immunol. 2023. PMID: 37153557 Free PMC article. Review.
References
-
- Atkinson C., Stewart S., Upton P.D., Machado R., Thomson J.R., Trembath R.C., Morrell N.W. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.CIR.0000012754.72951.3D. - DOI - PubMed
-
- Machado R.D., Southgate L., Eichstaedt C.A., Aldred M.A., Austin E.D., Best D.H., Chung W.K., Benjamin N., Elliott C.G., Eyries M., et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum. Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

