Haloferax mediterranei Cells as C50 Carotenoid Factories
- PMID: 33578828
- PMCID: PMC7916556
- DOI: 10.3390/md19020100
Haloferax mediterranei Cells as C50 Carotenoid Factories
Abstract
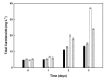
Haloarchaea produce C50 carotenoids such as bacterioruberin, which are of biotechnological in-terest. This study aimed to analyze the effect of different environmental and nutritional conditions on the cellular growth and dynamics of carotenoids accumulation in Haloferax mediterranei. The maximum production of carotenoids (40 µg·mL-1) was obtained during the stationary phase of growth, probably due to nutrient-limiting conditions (one-step culture). By seven days of culture, 1 mL culture produced 22.4 mg of dry weight biomass containing 0.18 % (w/w) of carotenoids. On the other hand, carbon-deficient cultures (low C/N ratio) were observed to be optimum for C50 bacterioruberin production by Hfx. mediterranei, but negatively affected the growth of cells. Thus, a two-steps process was evaluated for optimum carotenoids yield. In the first step, a nutri-ent-repleted culture medium enabled the haloarchaea to produce biomass, while in the second step, the biomass was incubated under osmotic stress and in a carbon-deficient medium. Under the conditions used, the obtained biomass contained 0.27% (w/w) of carotenoids after seven days, which accounts for 58.49 µg·mL-1 of carotenoids for a culture with turbidity 14.0.
Keywords: C/N ratio; Haloferax mediterranei; bacterioruberin; carotenoids; osmotic stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

