Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors
- PMID: 33578875
- PMCID: PMC7916554
- DOI: 10.3390/jcm10040685
Predicting the Risk of Postoperative Complications in Patients Undergoing Minimally Invasive Resection of Primary Liver Tumors
Abstract
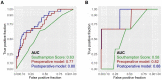
Minimal-invasive techniques are increasingly applied in clinical practice and have contributed towards improving postoperative outcomes. While comparing favorably with open surgery in terms of safety, the occurrence of severe complications remains a grave concern. To date, no objective predictive system has been established to guide clinicians in estimating complication risks as the relative contribution of general patient health, liver function and surgical parameters remain unclear. Here, we perform a single-center analysis of all consecutive patients undergoing laparoscopic liver resection for primary hepatic malignancies since 2010. Among the 210 patients identified, 32 developed major complications. Several independent predictors were identified through a multivariate analysis, defining a preoperative model: diabetes, history of previous hepatectomy, surgical approach, alanine aminotransferase levels and lesion entity. The addition of operative time and whether conversion was required significantly improved predictions and were thus incorporated into the postoperative model. Both models were able to identify patients with major complications with acceptable performance (area under the receiver-operating characteristic curve (AUC) for a preoperative model = 0.77 vs. postoperative model = 0.80). Internal validation was performed and confirmed the discriminatory ability of the models. An easily accessible online tool was deployed in order to estimate probabilities of severe complication without the need for manual calculation.
Keywords: cholangiocarcinoma; hepatocellular carcinoma; laparoscopic liver surgery; risk score.
Conflict of interest statement
The authors declare the following conflicts of interest unrelated to this work: Dominik Geisel—Bayer, Siemens, Uli Fehrenbach—Bayer, Siemens, GE, Wenzel Schöning—Merck, Bayer, Ethicon, Johann Pratschke—Merck, Medtronic, Intuitive, Verb Surgical, Moritz Schmelzle—Merck, Bayer, Erbe, Ethicon, Takeda, Olympus, Medtronic, Intuitive.
Figures

References
-
- Hilal M.A., Aldrighetti L., Dagher I., Edwin B., Troisi R.I., Alikhanov R., Aroori S., Belli G., Besselink M., Briceno J., et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018;268:11–18. doi: 10.1097/SLA.0000000000002524. - DOI - PubMed
-
- Lee D.H., Lee J.M., Lee J.Y., Kim S.H., Yoon J.H., Kim Y.J., Han J.K., Choi B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

