Microfluidic Organoids-on-a-Chip: Quantum Leap in Cancer Research
- PMID: 33578886
- PMCID: PMC7916612
- DOI: 10.3390/cancers13040737
Microfluidic Organoids-on-a-Chip: Quantum Leap in Cancer Research
Abstract
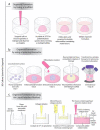
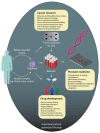
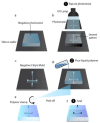
Organ-like cell clusters, so-called organoids, which exhibit self-organized and similar organ functionality as the tissue of origin, have provided a whole new level of bioinspiration for ex vivo systems. Microfluidic organoid or organs-on-a-chip platforms are a new group of micro-engineered promising models that recapitulate 3D tissue structure and physiology and combines several advantages of current in vivo and in vitro models. Microfluidics technology is used in numerous applications since it allows us to control and manipulate fluid flows with a high degree of accuracy. This system is an emerging tool for understanding disease development and progression, especially for personalized therapeutic strategies for cancer treatment, which provide well-grounded, cost-effective, powerful, fast, and reproducible results. In this review, we highlight how the organoid-on-a-chip models have improved the potential of efficiency and reproducibility of organoid cultures. More widely, we discuss current challenges and development on organoid culture systems together with microfluidic approaches and their limitations. Finally, we describe the recent progress and potential utilization in the organs-on-a-chip practice.
Keywords: living biobanks; microfluidics; organoids; organs-on-a-chip; tumoroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Engineering Microfluidic Organoid-on-a-Chip Platforms.Micromachines (Basel). 2019 Feb 27;10(3):165. doi: 10.3390/mi10030165. Micromachines (Basel). 2019. PMID: 30818801 Free PMC article.
-
Patient-Derived Organoids in Precision Medicine: Drug Screening, Organoid-on-a-Chip and Living Organoid Biobank.Front Oncol. 2021 Dec 30;11:762184. doi: 10.3389/fonc.2021.762184. eCollection 2021. Front Oncol. 2021. PMID: 35036354 Free PMC article. Review.
-
Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation.Adv Biol (Weinh). 2021 Jun;5(6):e2000024. doi: 10.1002/adbi.202000024. Epub 2021 Apr 15. Adv Biol (Weinh). 2021. PMID: 33856745 Free PMC article. Review.
-
Microfluidic organoids-on-a-chip: The future of human models.Semin Cell Dev Biol. 2023 Jul 30;144:41-54. doi: 10.1016/j.semcdb.2022.10.001. Epub 2022 Oct 11. Semin Cell Dev Biol. 2023. PMID: 36241560 Review.
-
Organoids-on-a-chip: microfluidic technology enables culture of organoids with enhanced tissue function and potential for disease modeling.Front Bioeng Biotechnol. 2025 Mar 11;13:1515340. doi: 10.3389/fbioe.2025.1515340. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40134772 Free PMC article. Review.
Cited by
-
Self-Therapeutic Cobalt Hydroxide Nanosheets (Co(OH)2 NS) for Ovarian Cancer Therapy.ACS Omega. 2021 Oct 19;6(43):28611-28619. doi: 10.1021/acsomega.1c03010. eCollection 2021 Nov 2. ACS Omega. 2021. PMID: 34746556 Free PMC article.
-
In vitro models for head and neck cancer: Current status and future perspective.Front Oncol. 2022 Aug 3;12:960340. doi: 10.3389/fonc.2022.960340. eCollection 2022. Front Oncol. 2022. PMID: 35992863 Free PMC article. Review.
-
Harness Organoid Models for Virological Studies in Animals: A Cross-Species Perspective.Front Microbiol. 2021 Sep 16;12:725074. doi: 10.3389/fmicb.2021.725074. eCollection 2021. Front Microbiol. 2021. PMID: 34603253 Free PMC article. Review.
-
Microfluidics as a promising technology for personalized medicine.Bioimpacts. 2024 Jun 16;15:29944. doi: 10.34172/bi.29944. eCollection 2025. Bioimpacts. 2024. PMID: 39963565 Free PMC article. Review.
-
Advances and Applications of Cancer Organoids in Drug Screening and Personalized Medicine.Stem Cell Rev Rep. 2024 Jul;20(5):1213-1226. doi: 10.1007/s12015-024-10714-6. Epub 2024 Mar 27. Stem Cell Rev Rep. 2024. PMID: 38532032 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

