Curdlan-Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration
- PMID: 33578913
- PMCID: PMC7916722
- DOI: 10.3390/polym13040526
Curdlan-Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration
Abstract
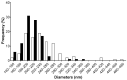
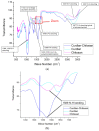
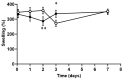
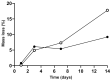
Polysaccharides have received a lot of attention in biomedical research for their high potential as scaffolds owing to their unique biological properties. Fibrillar scaffolds made of chitosan demonstrated high promise in tissue engineering, especially for skin. As far as bone regeneration is concerned, curdlan (1,3-β-glucan) is particularly interesting as it enhances bone growth by helping mesenchymal stem cell adhesion, by favoring their differentiation into osteoblasts and by limiting the osteoclastic activity. Therefore, we aim to combine both chitosan and curdlan polysaccharides in a new scaffold for bone regeneration. For that purpose, curdlan was electrospun as a blend with chitosan into a fibrillar scaffold. We show that this novel scaffold is biodegradable (8% at two weeks), exhibits a good swelling behavior (350%) and is non-cytotoxic in vitro. In addition, the benefit of incorporating curdlan in the scaffold was demonstrated in a scratch assay that evidences the ability of curdlan to express its immunomodulatory properties by enhancing cell migration. Thus, these innovative electrospun curdlan-chitosan scaffolds show great potential for bone tissue engineering.
Keywords: chitosan; curdlan; electrospinning; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier; Amsterdam, The Netherlands: 2004.
-
- Donglu S. Introduction to Biomaterials. Tsinghua University Press; Beijing, China: 2005.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

