From Stripes to a Beating Heart: Early Cardiac Development in Zebrafish
- PMID: 33578943
- PMCID: PMC7916704
- DOI: 10.3390/jcdd8020017
From Stripes to a Beating Heart: Early Cardiac Development in Zebrafish
Abstract
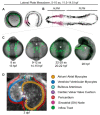
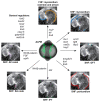
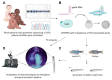
The heart is the first functional organ to form during vertebrate development. Congenital heart defects are the most common type of human birth defect, many originating as anomalies in early heart development. The zebrafish model provides an accessible vertebrate system to study early heart morphogenesis and to gain new insights into the mechanisms of congenital disease. Although composed of only two chambers compared with the four-chambered mammalian heart, the zebrafish heart integrates the core processes and cellular lineages central to cardiac development across vertebrates. The rapid, translucent development of zebrafish is amenable to in vivo imaging and genetic lineage tracing techniques, providing versatile tools to study heart field migration and myocardial progenitor addition and differentiation. Combining transgenic reporters with rapid genome engineering via CRISPR-Cas9 allows for functional testing of candidate genes associated with congenital heart defects and the discovery of molecular causes leading to observed phenotypes. Here, we summarize key insights gained through zebrafish studies into the early patterning of uncommitted lateral plate mesoderm into cardiac progenitors and their regulation. We review the central genetic mechanisms, available tools, and approaches for modeling congenital heart anomalies in the zebrafish as a representative vertebrate model.
Keywords: cardiovascular; cell fate; congenital heart disease; development; heart; lateral plate mesoderm; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

