Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid
- PMID: 33578971
- PMCID: PMC7916678
- DOI: 10.3390/biom11020260
Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid
Abstract
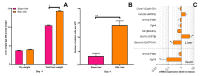
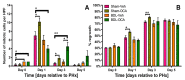
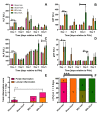
: In a previous study, obeticholic acid (OCA) increased liver growth before partial hepatectomy (PHx) in rats through the bile acid receptor farnesoid X-receptor (FXR). In that model, OCA was administered during obstructive cholestasis. However, patients normally undergo PHx several days after biliary drainage. The effects of OCA on liver regeneration were therefore studied in post-cholestatic Wistar rats. Rats underwent sham surgery or reversible bile duct ligation (rBDL), which was relieved after 7 days. PHx was performed one day after restoration of bile flow. Rats received 10 mg/kg OCA per day or were fed vehicle from restoration of bile flow until sacrifice 5 days after PHx. Liver regeneration was comparable between cholestatic and non-cholestatic livers in PHx-subjected rats, which paralleled liver regeneration a human validation cohort. OCA treatment induced ileal Fgf15 mRNA expression but did not enhance post-PHx hepatocyte proliferation through FXR/SHP signaling. OCA treatment neither increased mitosis rates nor recovery of liver weight after PHx but accelerated liver regrowth in rats that had not been subjected to rBDL. OCA did not increase biliary injury. Conclusively, OCA does not induce liver regeneration in post-cholestatic rats and does not exacerbate biliary damage that results from cholestasis. This study challenges the previously reported beneficial effects of OCA in liver regeneration in cholestatic rats.
Keywords: basolateral and canalicular transporters; bile acid metabolism; bile duct obstruction; bile salts; biliary obstruction; liver regeneration; partial hepatectomy; pharmacological intervention.
Conflict of interest statement
M.H. is the chief formulation officer at Nurish.Me LLC and Camelina Sun LTD and has equity in those companies (whose business activities are unrelated to the present work). There are no other conflict of interest.
Figures





References
-
- De Haan L., van der Lely S.J., Warps A.K., Hofsink Q., Olthof P.B., de Keijzer M.J., Lionarons D.A., Mendes-Dias L., Bruinsma B.G., Uygun K., et al. Post-hepatectomy liver regeneration in the context of bile acid homeostasis and the gutliver signaling axis. J. Clin. Transl. Res. 2018;4:1–46. doi: 10.18053/jctres.04.201801.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 10666/KWF Kankerbestrijding
- 12.2018/Zhejiang Provincial Foreign Expert Program Grant
- Z20H160031/Zhejiang Provincial Key Natural Science Foundation of China
- 12.2019/Grant for Jiaxing Key Laboratory for Photonanomedicine and Experimental Therapeutics
- MC_PC_12025/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

