Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review
- PMID: 33578987
- PMCID: PMC7916819
- DOI: 10.3390/biom11020258
Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review
Abstract
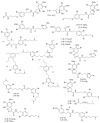
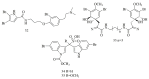
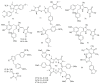
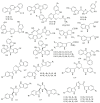
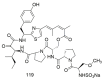
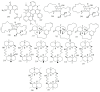
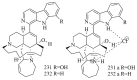
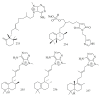
Marine sponges (porifera) have proved to be a prolific source of unique bioactive secondary metabolites, among which the alkaloids occupy a special place in terms of unprecedented structures and outstanding biological activities. Identification of active cytotoxic alkaloids extracted from marine animals, particularly sponges, is an important strive, due to lack of knowledge on traditional experiential and ethnopharmacology investigations. In this report, a comprehensive survey of demospongian bioactive alkaloids in the range 1987-2020 had been performed with a special emphasis on the potent cytotoxic activity. Different resources and databases had been investigated, including Scifinder (database for the chemical literature) CAS (Chemical Abstract Service) search, web of science, Marin Lit (marine natural products research) database. More than 230 representatives of different classes of alkaloids had been reviewed and classified, different genera belonging to the phylum porifera had been shown to be a prolific source of alkaloidal molecules, including Agelas sp., Suberea sp., Mycale sp., Haliclona sp., Epipolasis sp., Monanchora sp., Crambe sp., Reniera sp., and Xestospongia sp., among others. The sufficient production of alkaloids derived from sponges is a prosperous approach that requires more attention in future studies to consider the constraints regarding the supply of drugs, attained from marine organisms.
Keywords: alkaloids; cytotoxicity; marine drugs; secondary metabolites; sponges.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

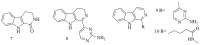
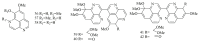




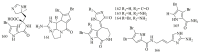




References
-
- Fattorusso E., Taglialatela-Scafati O. Modern Alkaloids: Structure, Isolation, Synthesis, And Biology. John Wiley & Sons; Hoboken, NJ, USA: 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

