Challenges and advances in clinical applications of mesenchymal stromal cells
- PMID: 33579329
- PMCID: PMC7880217
- DOI: 10.1186/s13045-021-01037-x
Challenges and advances in clinical applications of mesenchymal stromal cells
Abstract
Mesenchymal stromal cells (MSCs), also known as mesenchymal stem cells, have been intensely investigated for clinical applications within the last decades. However, the majority of registered clinical trials applying MSC therapy for diverse human diseases have fallen short of expectations, despite the encouraging pre-clinical outcomes in varied animal disease models. This can be attributable to inconsistent criteria for MSCs identity across studies and their inherited heterogeneity. Nowadays, with the emergence of advanced biological techniques and substantial improvements in bio-engineered materials, strategies have been developed to overcome clinical challenges in MSC application. Here in this review, we will discuss the major challenges of MSC therapies in clinical application, the factors impacting the diversity of MSCs, the potential approaches that modify MSC products with the highest therapeutic potential, and finally the usage of MSCs for COVID-19 pandemic disease.
Keywords: Artificial intelligence (AI); COVID-19; Clinical applications; Extracellular vesicles; Heterogeneity; Mesenchymal stromal cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
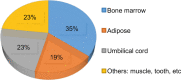
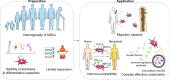
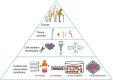

References
Publication types
MeSH terms
Grants and funding
- 2017YFE0131600/National Key Research and Development Project of China
- 81870121/National Natural Science Foundation of China
- 81671585/National Natural Science Foundation of China
- 82070176/National Natural Science Foundation of China
- 81700825/National Natural Science Foundation of China
- 2019B151502006/Natural Science Foundation of Guangdong Province
- 2019B020236004/Natural Science Foundation of Guangdong Province
- 201906010076/Science and Technology Program of Guangzhou
- 201803040005/Science and Technology Program of Guangzhou
- 2017B020230004/Science and Technology Planning Project of Guangdong Province
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

