Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome
- PMID: 33579698
- PMCID: PMC7880603
- DOI: 10.1126/sciadv.abe5085
Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome
Abstract
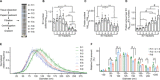
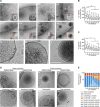
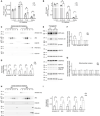
Mitochondrial dysfunction is an established hallmark of aging and neurodegenerative disorders such as Down syndrome (DS) and Alzheimer's disease (AD). Using a high-resolution density gradient separation of extracellular vesicles (EVs) isolated from murine and human DS and diploid control brains, we identify and characterize a previously unknown population of double-membraned EVs containing multiple mitochondrial proteins distinct from previously described EV subtypes, including microvesicles and exosomes. We term these newly identified mitochondria-derived EVs "mitovesicles." We demonstrate that brain-derived mitovesicles contain a specific subset of mitochondrial constituents and that their levels and cargo are altered during pathophysiological processes where mitochondrial dysfunction occurs, including in DS. The development of a method for the selective isolation of mitovesicles paves the way for the characterization in vivo of biological processes connecting EV biology and mitochondria dynamics and for innovative therapeutic and diagnostic strategies.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Gauthier S. A., Pérez-González R., Sharma A., Huang F.-K., Alldred M. J., Pawlik M., Kaur G., Ginsberg S. D., Neubert T. A., Levy E., Enhanced exosome secretion in Down syndrome brain - A protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 5, 65 (2017). - PMC - PubMed
-
- Puhm F., Afonyushkin T., Resch U., Obermayer G., Rohde M., Penz T., Schuster M., Wagner G., Rendeiro A. F., Melki I., Kaun C., Wojta J., Bock C., Jilma B., Mackman N., Boilard E., Binder C. J., Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ. Res. 125, 43–52 (2019). - PubMed
-
- Jang S. C., Crescitelli R., Cvjetkovic A., Belgrano V., Olofsson Bagge R., Sundfeldt K., Ochiya T., Kalluri R., Lötvall J., Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 8, 1635420 (2019). - PMC - PubMed
-
- Guescini M., Genedani S., Stocchi V., Agnati L. F., Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. (Vienna) 117, 1 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

