Copan eNAT Transport System To Address Challenges in COVID-19 Diagnostics in Regions with Limited Testing Access
- PMID: 33579730
- PMCID: PMC8091855
- DOI: 10.1128/JCM.00110-21
Copan eNAT Transport System To Address Challenges in COVID-19 Diagnostics in Regions with Limited Testing Access
Abstract
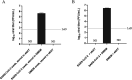
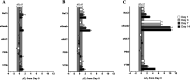
Community-based health care clinics and hospital outreach services have the potential to expand coronavirus disease 2019 (COVID-19) diagnostics to rural areas. However, reduced specimen stability during extended transport, the absence of a cold chain to centralized laboratories, and biosafety concerns surrounding specimen handling have limited this expansion. In the following study, we evaluated eNAT (Copan Italia, Brescia, Italy) as an alternative transport system to address the biosafety and stability challenges associated with expanding COVID-19 diagnostics to rural and remote regions. In this study, we demonstrated that high-titer severe acute respiratory virus syndrome coronavirus 2 (SARS-CoV-2) lysate placed into eNAT medium cannot be propagated in cell culture, supporting viral inactivation. To account for off-site testing in these settings, we assessed the stability of contrived nasopharyngeal (NP) specimens stored for up to 14 days in various transport media (eNAT, eSwab, viral transport medium [VTM], saline, and phosphate-buffered saline [PBS]) at 4°C, 22 to 25°C, and 35°C. The molecular detection of SARS-CoV-2 was unaffected by sample storage temperature over the 2 weeks when stored in eNAT or PBS (change in cycle threshold, ≤1). In contrast, variable stability was observed across test conditions for other transport media. As eNAT can inactivate SARS-CoV-2, it may support COVID-19 diagnostics at the point of care. Evaluation of compatibility of eNAT with Cepheid Xpert Xpress SARS-CoV-2 assay demonstrated diagnostic accuracy and sensitivity equivalent to those of VTM. Taken together, these findings suggest that the implementation of eNAT as a collection device can expand COVID-19 testing to areas with limited health care access.
Keywords: COVID-19; Cepheid Xpert Xpress SARS-CoV-2; SARS-CoV-2; SARS-CoV-2 inactivation; SARS-CoV-2 specimen stability; eNAT; limited testing access; low-resource settings; point of care; rural healthcare.
Copyright © 2021 Richard-Greenblatt et al.
Figures
References
-
- Carter C, Thi Lan Anh N, Notter J. 2020. COVID-19 disease: perspectives in low- and middle-income countries. Clinics Integrated Care 1:100005. doi: 10.1016/j.intcar.2020.100005. - DOI
-
- van Bockel D, Munier CML, Turville S, Badman SG, Walker G, Stella AO, Aggarwal A, Yeang M, Condylios A, Kelleher AD, Applegate TL, Vallely A, Whiley D, Rawlinson W, Cunningham P, Kaldor J, Guy R. 2020. Evaluation of commercially available viral transport medium (VTM) for SARS-CoV-2 inactivation and use in point-of-care (POC) testing. Viruses 12:1208. doi: 10.3390/v12111208. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

