Assessing the efficacy, safety and utility of closed-loop insulin delivery compared with sensor-augmented pump therapy in very young children with type 1 diabetes (KidsAP02 study): an open-label, multicentre, multinational, randomised cross-over study protocol
- PMID: 33579766
- PMCID: PMC7883854
- DOI: 10.1136/bmjopen-2020-042790
Assessing the efficacy, safety and utility of closed-loop insulin delivery compared with sensor-augmented pump therapy in very young children with type 1 diabetes (KidsAP02 study): an open-label, multicentre, multinational, randomised cross-over study protocol
Abstract
Introduction: Diabetes management in very young children remains challenging. Glycaemic targets are achieved at the expense of high parental diabetes management burden and frequent hypoglycaemia, impacting quality of life for the whole family. Our objective is to assess whether automated insulin delivery can improve glycaemic control and alleviate the burden of diabetes management in this particular age group.
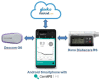
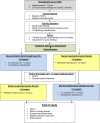
Methods and analysis: The study adopts an open-label, multinational, multicentre, randomised, crossover design and aims to randomise 72 children aged 1-7 years with type 1 diabetes on insulin pump therapy. Following screening, participants will receive training on study insulin pump and study continuous glucose monitoring devices. Participants will be randomised to 16-week use of the hybrid closed-loop system (intervention period) or to 16-week use of sensor-augmented pump therapy (control period) with 1-4 weeks washout period before crossing over to the other arm. The order of the two study periods will be random. The primary endpoint is the between-group difference in time spent in the target glucose range from 3.9 to 10.0 mmol/L based on sensor glucose readings during the 16-week study periods. Analyses will be conducted on an intention-to-treat basis. Key secondary endpoints are between group differences in time spent above and below target glucose range, glycated haemoglobin and average sensor glucose. Participants' and caregivers' experiences will be evaluated using questionnaires and qualitative interviews, and sleep quality will be assessed. A health economic analysis will be performed.
Ethics and dissemination: Ethics approval has been obtained from Cambridge East Research Ethics Committee (UK), Ethics Committees of the University of Innsbruck, the University of Vienna and the University of Graz (Austria), Ethics Committee of the Medical Faculty of the University of Leipzig (Germany) and Comité National d'Ethique de Recherche (Luxembourg). The results will be disseminated by peer-reviewed publications and conference presentations.
Trial registration number: NCT03784027.
Keywords: diabetes & endocrinology; general diabetes; paediatric endocrinology; paediatrics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SEH declares speaker honoraria from Eli Lilly, Pfizer and Sanofi. EF-R reports having received speaker honoraria from Minimed Medtronic and Eli Lilly, serving on advisory boards for Eli Lilly. TMK has received speaker honoraria from Minimed Medtronic, Roche and Eli Lilly and consulted Sanofi-Aventis. CdB has received speaker honoraria from Minimed Medtronic, and is member of their European Psychology Advisory Board. BR-M reports having received speaker honoraria from Abbott, Minimed Medtronic, Eli Lilly, Roche, Menarini and Novo Nordisk, serving on advisory boards for Eli Lilly. FC has received speaking fees from Abbott, Medtronic and Eli Lilly. RH reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk; receiving license fees from BBraun and Medtronic; having served as a consultant to BBraun, patents and patent applications related to closed-loop insulin delivery, and director at CamDiab. JKM is shareholder of Decide Clinical Software, a member in the advisory board of Boehringer Ingelheim, Eli Lilly, Medtronic, Prediktor A/S, Roche Diabetes Care, Sanofi-Aventis and received speaker honoraria from Abbott Diabetes Care, Astra Zeneca, Dexcom, Eli Lilly, NovoNordisk A/S, Roche Diabetes Care, Servier and Takeda.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
