METTL3-dependent N6-methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium
- PMID: 33579825
- PMCID: PMC7896299
- DOI: 10.1073/pnas.2025070118
METTL3-dependent N6-methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium
Abstract
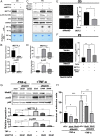
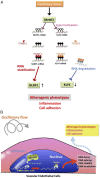
Atherosclerosis is characterized by the plaque formation that restricts intraarterial blood flow. The disturbed blood flow with the associated oscillatory stress (OS) at the arterial curvatures and branch points can trigger endothelial activation and is one of the risk factors of atherosclerosis. Many studies reported the mechanotransduction related to OS and atherogenesis; however, the transcriptional and posttranscriptional regulatory mechanisms of atherosclerosis remain unclear. Herein, we investigated the role of N6-methyladenosine (m6A) RNA methylation in mechanotransduction in endothelial cells (ECs) because of its important role in epitranscriptome regulation. We have identified m6A methyltransferase METTL3 as a responsive hub to hemodynamic forces and atherogenic stimuli in ECs. OS led to an up-regulation of METTL3 expression, accompanied by m6A RNA hypermethylation, increased NF-κB p65 Ser536 phosphorylation, and enhanced monocyte adhesion. Knockdown of METTL3 abrogated this OS-induced m6A RNA hypermethylation and other manifestations, while METTL3 overexpression led to changes resembling the OS effects. RNA-sequencing and m6A-enhanced cross-linking and immunoprecipitation (eCLIP) experiments revealed NLRP1 and KLF4 as two hemodynamics-related downstream targets of METTL3-mediated hypermethylation. The METTL3-mediated RNA hypermethylation up-regulated NLRP1 transcript and down-regulated KLF4 transcript through YTHDF1 and YTHDF2 m6A reader proteins, respectively. In the in vivo atherosclerosis model, partial ligation of the carotid artery led to plaque formation and up-regulation of METTL3 and NLRP1, with down-regulation of KLF4; knockdown of METTL3 via repetitive shRNA administration prevented the atherogenic process, NLRP3 up-regulation, and KLF4 down-regulation. Collectively, we have demonstrated that METTL3 serves a central role in the atherogenesis induced by OS and disturbed blood flow.
Keywords: METTL3; N6-methyladeosine RNA methylation; atherosclerosis; oscillatory flow; shear stress.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Neth P., Nazari-Jahantigh M., Schober A., Weber C., MicroRNAs in flow-dependent vascular remodelling. Cardiovasc. Res. 99, 294–303 (2013). - PubMed
-
- Geula S., et al. , Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

