T Cells Limit Accumulation of Aggregate Pathology Following Intrastriatal Injection of α-Synuclein Fibrils
- PMID: 33579871
- PMCID: PMC8150548
- DOI: 10.3233/JPD-202351
T Cells Limit Accumulation of Aggregate Pathology Following Intrastriatal Injection of α-Synuclein Fibrils
Abstract
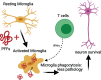
Background: α-Synuclein (α-syn) is the predominant protein in Lewy-body inclusions, which are pathological hallmarks of α-synucleinopathies, such as Parkinson's disease (PD) and multiple system atrophy (MSA). Other hallmarks include activation of microglia, elevation of pro-inflammatory cytokines, as well as the activation of T and B cells. These immune changes point towards a dysregulation of both the innate and the adaptive immune system. T cells have been shown to recognize epitopes derived from α-syn and altered populations of T cells have been found in PD and MSA patients, providing evidence that these cells can be key to the pathogenesis of the disease.ObjectiveTo study the role of the adaptive immune system with respect to α-syn pathology.
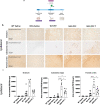
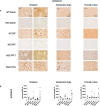
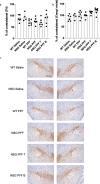
Methods: We injected human α-syn preformed fibrils (PFFs) into the striatum of immunocompromised mice (NSG) and assessed accumulation of phosphorylated α-syn pathology, proteinase K-resistant α-syn pathology and microgliosis in the striatum, substantia nigra and frontal cortex. We also assessed the impact of adoptive transfer of naïve T and B cells into PFF-injected immunocompromised mice.
Results: Compared to wildtype mice, NSG mice had an 8-fold increase in phosphorylated α-syn pathology in the substantia nigra. Reconstituting the T cell population decreased the accumulation of phosphorylated α-syn pathology and resulted in persistent microgliosis in the striatum when compared to non-transplanted mice.
Conclusion: Our work provides evidence that T cells play a role in the pathogenesis of experimental α-synucleinopathy.
Keywords: Parkinson’s disease; T lymphocytes; alpha-synuclein; microglia; multiple system atrophy; phosphorylated alpha-synuclein.
Conflict of interest statement
PB has received commercial support as a consultant from Axial Biotherapeutics, Calico, CuraSen, Fujifilm-Cellular Dynamics International, Idorsia, IOS Press Partners, LifeSci Capital LLC, Lundbeck A/S and Living Cell Technologies LTD. He has received commercial support for grants/research from Lundbeck A/S and Roche. He has ownership interests in Acousort AB and Axial Biotherapeutics and is on the steering committee of the NILO-PD trial. The authors declare no additional competing financial interests.
Figures



References
-
- Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18, S210–S212. - PubMed
-
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H (1998) α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 249, 180–182. - PubMed
-
- Rydbirk R, Elfving B, Andersen MD, Langbøl MA, Folke J, Winge K, Pakkenberg B, Brudek T, Aznar S (2017) Cytokine profiling in the prefrontal cortex of Parkinson’s Disease and Multiple System Atrophy patients. Neurobiol Dis 106, 269–278. - PubMed
-
- Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J (2020) Parkinson disease and the immune system —associations, mechanisms and therapeutics. Nat Rev Neurol 16, 303–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

