Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient
- PMID: 33580193
- PMCID: PMC7880994
- DOI: 10.1038/s41698-021-00149-4
Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient
Abstract
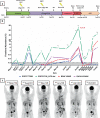
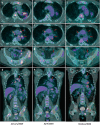
The survival outcomes of the FLAURA trial support osimertinib as the new standard of care for untreated patients harboring activating mutations in the epidermal growth factor receptor (EGFR). Despite the initial response, disease progression invariably occurs. Although uncommon, BRAF V600E mutation arises as a unique mechanism of resistance, and thus far, no prospective studies are available to support concurrent EGFR/BRAF blockade. We report a case of impressive radiological and ctDNA response under dabrafenib, trametinib, and osimertinib in an advanced EGFR-mutant lung adenocarcinoma patient who developed BRAF V600E as one of the acquired resistance mechanisms to second-line osimertinib. Moreover, the patient experienced remarkable clinical improvement and good tolerance to combination therapy. The present case suggests the importance of prospective studies evaluating both efficacy and safety of the combination in later line settings and points towards the potential of ctDNA to monitor resistance mechanisms and treatment benefit in clinical practice.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

