Intravenous ferric derisomaltose for the treatment of iron deficiency anemia
- PMID: 33580972
- PMCID: PMC8248147
- DOI: 10.1002/ajh.26124
Intravenous ferric derisomaltose for the treatment of iron deficiency anemia
Abstract
Intravenous (IV) iron is the therapy of choice when oral iron is ineffective or poorly tolerated, yet use has been limited by fears of hypersensitivity reactions (HSRs). Newer formulations that bind iron more tightly and release it more slowly have made the risk of serious or severe HSRs very low. One such formulation, ferric derisomaltose, has been approved in the United States for delivery of 1000 mg iron in a single IV infusion. Ferric derisomaltose rapidly repletes iron parameters with low rates of serious or severe HSRs. Single-infusion iron repletion offers convenience, eliminates adherence concerns, and reduces healthcare resource utilization.
© 2021 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
M.A. reports grants from AMAG Pharmaceuticals and non‐promotional educational talks for Pfizer and Pharmacosmos. T.D. and D.H. have nothing to disclose.
Figures
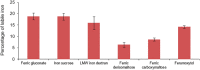
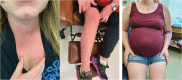
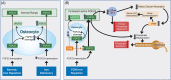
References
-
- Goetsch AT, Moore CV, Minnich V. Observations on the effect of massive doses of iron given intravenously to patients with hypochromic anemia. Blood. 1946;1:129‐142. - PubMed
-
- Sheet TP. Fisons’ opticrom, imferon may be off U.S. market until late 1992 as the company upgrades U.K. manufacturing plant to meet FDA quality control concerns. Informa Pharm Intellig. 1991. https://pink.pharmaintelligence.informa.com/PS020190/FISONS‐OPTICROM‐IMF.... Accessed February 17, 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
