Saliva is a reliable and accessible source for the detection of SARS-CoV-2
- PMID: 33581365
- PMCID: PMC7876483
- DOI: 10.1016/j.ijid.2021.02.009
Saliva is a reliable and accessible source for the detection of SARS-CoV-2
Abstract
Objectives: The aim of this study was to investigate the feasibility of saliva sampling as a non-invasive and safer tool to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and to compare its reproducibility and sensitivity with nasopharyngeal swab samples (NPS). The use of sample pools was also investigated.
Methods: A total of 2107 paired samples were collected from asymptomatic healthcare and office workers in Mexico City. Sixty of these samples were also analyzed in two other independent laboratories for concordance analysis. Sample processing and analysis of virus genetic material were performed according to standard protocols described elsewhere. A pooling analysis was performed by analyzing the saliva pool and the individual pool components.
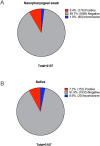
Results: The concordance between NPS and saliva results was 95.2% (kappa 0.727, p = 0.0001) and 97.9% without considering inconclusive results (kappa 0.852, p = 0.0001). Saliva had a lower number of inconclusive results than NPS (0.9% vs 1.9%). Furthermore, saliva showed a significantly higher concentration of both total RNA and viral copies than NPS. Comparison of our results with those of the other two laboratories showed 100% and 97% concordance. Saliva samples are stable without the use of any preservative, and a positive SARS-CoV-2 sample can be detected 5, 10, and 15 days after collection when the sample is stored at 4 °C.
Conclusions: The study results indicate that saliva is as effective as NPS for the identification of SARS-CoV-2-infected asymptomatic patients. Sample pooling facilitates the analysis of a larger number of samples, with the benefit of cost reduction.
Keywords: COVID-19; Diagnostic test; Pooling strategy; SARS-CoV-2; Saliva testing.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Centers for Disease Control and Prevention . 2020. (2019-nCoV), CDC 2019-Novel Coronavirus Panel, Real-Time RT-PCR Diagnostic.https://www.fda.gov/media/134922/download
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

