Irisin deletion induces a decrease in growth and fertility in mice
- PMID: 33581723
- PMCID: PMC7881587
- DOI: 10.1186/s12958-021-00702-7
Irisin deletion induces a decrease in growth and fertility in mice
Abstract
Background: Irisin, which is cleaved from fibronectin type III domain-containing protein 5 (Fndc5), plays an important role in energy homeostasis. The link between energy metabolism and reproduction is well known. However, the biological actions of irisin in reproduction remain largely unexplored.
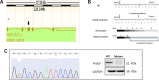
Methods: In this study, we generated Fndc5 gene mutation to create irisin deficient mice. Female wild-type (WT) and Fndc5 mutant mice were fed with standard chow for 48 weeks. Firstly, the survival rate, body weight and fertility were described in mice. Secondly, the levels of steroid hormones in serum were measured by ELISA, and the estrus cycle and the appearance of follicles were determined by vaginal smears and ovarian continuous sections. Thirdly, mRNA-sequencing analysis was used to compare gene expression between the ovaries of Fndc5 mutant mice and those of WT mice. Finally, the effects of exogenous irisin on steroid hormone production was investigated in KGN cells.
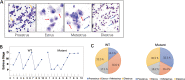
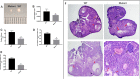
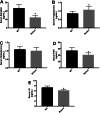
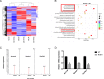
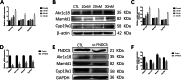
Results: The mice lacking irisin presented increased mortality, reduced body weight and poor fertility. Analysis of sex hormones showed decreased levels of estradiol, follicle-stimulating hormone and luteinizing hormone, and elevated progesterone levels in Fndc5 mutant mice. Irisin deficiency in mice was associated with irregular estrus, reduced ratio of antral follicles. The expressions of Akr1c18, Mamld1, and Cyp19a1, which are involved in the synthesis of steroid hormones, were reduced in the ovaries of mutant mice. Exogenous irisin could promote the expression of Akr1c18, Mamld1, and Cyp19a1 in KGN cells, stimulating estradiol production and inhibiting progesterone secretion.
Conclusions: Irisin deficiency was related to disordered endocrinology metabolism in mice. The irisin deficient mice showed poor growth and development, and decreased fertility. Irisin likely have effects on the expressions of Akr1c18, Mamld1 and Cyp19a1 in ovary, regulating the steroid hormone production. This study provides novel insights into the potential role of irisin in mammalian growth and reproduction.
Keywords: Growth and development; Hormone metabolism; Irisin; Reproduction.
Conflict of interest statement
The authors declare no competing interest.
Figures

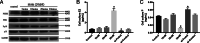
Similar articles
-
Irisin modulates glucose metabolism and inhibits steroidogenesis in bovine granulosa cells.Reproduction. 2023 Mar 30;165(5):533-542. doi: 10.1530/REP-22-0404. Print 2023 May 1. Reproduction. 2023. PMID: 36795655
-
NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin.Theranostics. 2021 Feb 25;11(9):4381-4402. doi: 10.7150/thno.53652. eCollection 2021. Theranostics. 2021. PMID: 33754067 Free PMC article.
-
Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells.Biol Reprod. 2008 Dec;79(6):1074-83. doi: 10.1095/biolreprod.108.069435. Epub 2008 Aug 13. Biol Reprod. 2008. PMID: 18703422 Free PMC article.
-
Irisin and the fibronectin type III domain-containing family: structure, signaling and role in female reproduction.Reproduction. 2022 May 23;164(1):R1-R9. doi: 10.1530/REP-22-0037. Reproduction. 2022. PMID: 35521900 Review.
-
Insulin-like peptide 3 (INSL3) is a major regulator of female reproductive physiology.Hum Reprod Update. 2018 Nov 1;24(6):639-651. doi: 10.1093/humupd/dmy029. Hum Reprod Update. 2018. PMID: 30204868 Review.
Cited by
-
Central Irisin Signaling Is Required for Normal Timing of Puberty in Female Mice.Endocrinology. 2022 Dec 19;164(2):bqac208. doi: 10.1210/endocr/bqac208. Endocrinology. 2022. PMID: 36503981 Free PMC article.
-
Irisin reduces the abnormal reproductive and metabolic phenotypes of PCOS by regulating the activity of brown adipose tissue in mice†.Biol Reprod. 2022 Oct 11;107(4):1046-1058. doi: 10.1093/biolre/ioac125. Biol Reprod. 2022. PMID: 35713297 Free PMC article.
-
Irisin: circulating levels in serum and its relation to gonadal axis.Endocrine. 2022 Mar;75(3):663-671. doi: 10.1007/s12020-022-02981-5. Epub 2022 Jan 17. Endocrine. 2022. PMID: 35040046 Free PMC article. Review.
-
The effects of irisin and leptin on steroidogenic enzyme gene expression in human granulosa cells: In vitro studies.Metabol Open. 2023 Jan 13;17:100230. doi: 10.1016/j.metop.2023.100230. eCollection 2023 Mar. Metabol Open. 2023. PMID: 36686605 Free PMC article.
-
Irisin, Energy Homeostasis and Male Reproduction.Front Physiol. 2021 Sep 21;12:746049. doi: 10.3389/fphys.2021.746049. eCollection 2021. Front Physiol. 2021. PMID: 34621189 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

