Continuous use of glycomacropeptide in the nutritional management of patients with phenylketonuria: a clinical perspective
- PMID: 33581730
- PMCID: PMC7881530
- DOI: 10.1186/s13023-021-01721-8
Continuous use of glycomacropeptide in the nutritional management of patients with phenylketonuria: a clinical perspective
Abstract
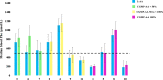
Background: In phenylketonuria (PKU), modified casein glycomacropeptide supplements (CGMP-AA) are used as an alternative to the traditional phenylalanine (Phe)-free L-amino acid supplements (L-AA). However, studies focusing on the long-term nutritional status of CGMP-AA are lacking. This retrospective study evaluated the long-term impact of CGMP-AA over a mean of 29 months in 11 patients with a mean age at CGMP-AA onset of 28 years (range 15-43) [8 females; 2 hyperphenylalaninaemia (HPA), 3 mild PKU, 3 classical PKU and 3 late-diagnosed]. Outcome measures included metabolic control, anthropometry, body composition and biochemical parameters.
Results: CGMP-AA, providing 66% of protein equivalent intake from protein substitute, was associated with no significant change in blood Phe with CGMP-AA compared with baseline (562 ± 289 µmol/L vs 628 ± 317 µmol/L; p = 0.065). In contrast, blood tyrosine significantly increased on CGMP-AA (52.0 ± 19.2 μmol/L vs 61.4 ± 23.8 μmol/L; p = 0.027).
Conclusions: Biochemical nutritional markers remained unchanged which is an encouraging finding in adults with PKU, many of whom are unable to maintain full adherence with nutritionally fortified protein substitutes. Longitudinal, prospective studies with larger sample sizes are necessary to fully understand the metabolic impact of using CGMP-AA in PKU.
Keywords: Amino acids; Casein glycomacropeptide; Nutritional status; Phenylalanine; Phenylketonuria; Tyrosine.
Conflict of interest statement
A.P. has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Nutricia, Merck Serono, BioMarin, and Mevalia to attend scientific meetings. M.F.A. received grants from Glutamine, Nutricia, Merck Serono, BioMarin, Orphan, and Lifediet to attend congress and for education. A.M. has received research funding and honoraria from Nutricia, Vitaflo International, BioMarin, Mevalia, and Pharma Galen. She is a member of the European Nutrition Expert Panel (BioMarin), and a member of the following advisory boards: the European PKU Group Board (BioMarin), Element (Danone-Nutricia), Excemed, Arla, and Applied Pharma Research. J.C.R. is member of the European Nutrition Expert Panel (BioMarin) and of the advisory boards of Applied Pharma Research and Nutricia. He has received speaker’s fees from Applied Pharma Research, Merck Serono, BioMarin, Nutricia, Vitaflo, Cambrooke, PIAM, and Lifediet.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

