Functional phenotyping of the CYP2D6 probe drug codeine in the horse
- PMID: 33581736
- PMCID: PMC7881596
- DOI: 10.1186/s12917-021-02788-y
Functional phenotyping of the CYP2D6 probe drug codeine in the horse
Abstract
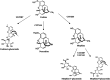
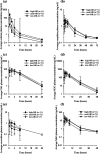
Background: In humans, the drug metabolizing enzyme CYP2D6 is highly polymorphic resulting in substantial differences in the metabolism of drugs including anti-arrhythmics, neuroleptics, and opioids. The objective of this study was to phenotype a population of 100 horses from five different breeds and assess differences in the metabolic activity of the equine CYP2D6 homolog using codeine as a probe drug. Administration of a probe drug is a common method used for patient phenotyping in human medicine, whereby the ratio of parent drug to metabolite (metabolic ratio, MR) can be used to compare relative enzyme function between individuals. A single oral dose of codeine (0.6 mg/kg) was administered and plasma concentrations of codeine and its metabolites were determined using liquid chromatography mass spectrometry. The MR of codeine O-demethylation [(codeine)/(morphine + morphine-3-glucuronide + morphine-6-glucuronide)] was determined using the area under the plasma concentration-time curve extrapolated from time zero to infinity (AUC0-∞) for each analyte and used to group horses into predicted phenotypes (high-, moderate-, and low-MR).
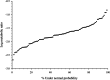
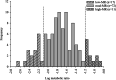
Results: The MR of codeine O-demethylation ranged from 0.002 to 0.147 (median 0.018) among all horses. No significant difference in MR was observed between breeds, age, or sex. Of the 100 horses, 11 were classified as high-MR, 72 moderate-MR, and 17 low-MR. Codeine AUC0-∞ and O-demethylation MR were significantly different (p < 0.05) between all three groups. The mean ± SD MR was 0.089 ± 0.027, 0.022 ± 0.011, and 0.0095 ± 0.001 for high-, moderate-, and low-MR groups, respectively. The AUC for the morphine metabolites morphine-3-glucuronide and morphine-6-glucuronide were significantly different between high-and low-MR groups (p < 0.004 and p < 0.006).
Conclusions: The MR calculated from plasma following codeine administration allowed for classification of horses into metabolic phenotypes within a large population. The range of codeine metabolism observed among horses suggests the presence of genetic polymorphisms in CYP2D82 of which codeine is a known substrate. Additional studies including CYP2D82 genotyping of high- and low-MR individuals are necessary to determine the presence of CYP2D polymorphisms and their functional implications with respect to the metabolism of therapeutics.
Keywords: CYP450; Codeine; Horse; Metabolic ratio; Phenotyping.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
Characterization of the in vitro CYP450 mediated metabolism of the polymorphic CYP2D6 probe drug codeine in horses.Biochem Pharmacol. 2019 Oct;168:184-192. doi: 10.1016/j.bcp.2019.07.005. Epub 2019 Jul 8. Biochem Pharmacol. 2019. PMID: 31295464
-
The impact of CYP2D6 polymorphisms on the pharmacokinetics of codeine and its metabolites in Mongolian Chinese subjects.Eur J Clin Pharmacol. 2014 Jan;70(1):57-63. doi: 10.1007/s00228-013-1573-x. Eur J Clin Pharmacol. 2014. PMID: 24077935 Clinical Trial.
-
Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication.Pharmacogenomics J. 2007 Aug;7(4):257-65. doi: 10.1038/sj.tpj.6500406. Epub 2006 Jul 4. Pharmacogenomics J. 2007. PMID: 16819548 Clinical Trial.
-
The pharmacogenetics of codeine hypoalgesia.Pharmacogenetics. 1995 Dec;5(6):335-46. doi: 10.1097/00008571-199512000-00001. Pharmacogenetics. 1995. PMID: 8845855 Review.
-
Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I.Clin Pharmacokinet. 2009;48(11):689-723. doi: 10.2165/11318030-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19817501 Review.
Cited by
-
Pharmacokinetics, adverse effects and effects on thermal nociception following administration of three doses of codeine to horses.BMC Vet Res. 2022 May 25;18(1):196. doi: 10.1186/s12917-022-03299-0. BMC Vet Res. 2022. PMID: 35614473 Free PMC article.
-
Preliminary investigation of potential links between pigmentation variants and opioid analgesic effectiveness in horses during cerebrospinal fluid centesis.BMC Vet Res. 2024 Jul 12;20(1):311. doi: 10.1186/s12917-024-04139-z. BMC Vet Res. 2024. PMID: 38997753 Free PMC article.
-
Genetic analysis of the equine orthologues for human CYP2D6: unraveling the complexity of the CYP2D family in horses.Front Vet Sci. 2023 Oct 19;10:1188633. doi: 10.3389/fvets.2023.1188633. eCollection 2023. Front Vet Sci. 2023. PMID: 37929279 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

