Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study
- PMID: 33581749
- PMCID: PMC9059427
- DOI: 10.1016/S2352-4642(21)00020-1
Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study
Abstract
Background: Cisplatin is used to treat a wide range of childhood cancers and cisplatin-induced hearing loss (CIHL) is a common and debilitating toxicity. We aimed to address persistent knowledge gaps in CIHL by establishing benchmarks for the prevalence of and risk factors for CIHL.
Methods: In this multi-institutional cohort study, children (age 0-14 years), adolescents, and young adults (age 15-39 years) diagnosed with a cisplatin-treated tumour from paediatric cancer centres, who had available cisplatin dosing information, and primary audiology data for central review from consortia located in Canada and the USA were eligible for inclusion. Audiology was centrally reviewed and CIHL graded using the consensus International Society of Pediatric Oncology (SIOP) Boston Ototoxicity Scale. We assessed the prevalence of moderate or severe CIHL (SIOP grade ≥2) at latest follow-up and end of therapy, in each demographic, diagnosis, and treatment group and their relative contributions to risk for CIHL. Secondary endpoints explored associations of cisplatin dose reductions and CIHL with survival. We also examined whether cisplatin dose reductions and CIHL were associated with survival outcomes.
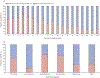
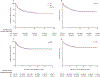
Findings: We included 1481 patients who received cisplatin. Of the 1414 (95·5%) participants who had audiometry at latest follow-up (mean 3·9 years [SD 4·2] since diagnosis), 620 (43·8%) patients developed moderate or severe CIHL. The highest prevalence of CIHL was seen in the youngest patients (aged <5 years; 360 [59·4%] of 606 patients) and those with a CNS tumour (221 [50·9%] of 434 patients), hepatoblastoma (110 [65·9%] of 167 patients), or neuroblastoma (154 [62·1%] of 248 patients). After accounting for cumulative cisplatin dose, higher fractionated doses were associated with risk for CIHL (for each 10mg/m2 increase per day, adjusted odds ratio [aOR] 1·15 [95% CI 1·07-1·25]; for each 50 mg/m2 increase per cycle aOR 2·16 [1·37-3·51]). Vincristine exposure was newly identified as a risk factor for CIHL (aOR 3·55 [2·19-5·84]). Dose reductions and moderate or severe CIHL were not significantly associated with survival differences.
Interpretation: Using this large, multicentre cohort, benchmarks were established for the prevalence of CIHL in patients treated with cisplatin. Variations in cisplatin dosing confer additive risk for developing CIHL and warrant investigation as a potential approach to decrease the burden of therapy.
Funding: US National Institutes of Health and National Institute on Deafness and Other Communication Disorders, US National Institutes of Health and National Cancer institute, St Baldrick's Foundation, Genome Canada, Genome British Columbia, Canadian Institutes of Health Research, the Canada Foundation for Innovation, University of British Columbia, British Columbia Children's Hospital Research Institute, British Columbia Provincial Health Services Authority, Health Canada, and C17 Research Network.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Hearing loss in childhood cancer survivors.Lancet Child Adolesc Health. 2021 May;5(5):e17. doi: 10.1016/S2352-4642(21)00099-7. Lancet Child Adolesc Health. 2021. PMID: 33864745 No abstract available.
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute, 2019.
-
- Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355(15): 1572–82. - PubMed
-
- Clemens E, van den Heuvel-Eibrink MM, Mulder RL, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol 2019; 20(1): e29–e41. - PMC - PubMed
-
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 2005; 23(34): 8588–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

