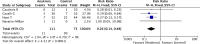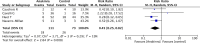Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies
- PMID: 33581979
- PMCID: PMC7862887
- DOI: 10.1016/j.ejim.2021.01.016
Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies
Abstract
Introduction: Severe COVID-19 cases have a detrimental hyper-inflammatory host response and different cytokine-blocking biologic agents were explored to improve outcomes. Anakinra blocks the activity of both IL-1α and IL‑1β and is approved for different autoinflammatory disorders, but it is used off-label for conditions characterized by an excess of cytokine production. Several studies on anakinra in COVID-19 patients reported positive effects. We performed a meta-analysis of all published evidence on the use of anakinra in COVID19 to investigate its effect on survival and need for mechanical ventilation.
Methods: We searched for any study performed on adult patients with acute hypoxemic failure related to 2019-nCoV infection, receiving anakinra versus any comparator. Primary endpoint was mortality at the longest available follow-up. Adverse effects, need for mechanical ventilation and discharge at home with no limitations were also analysed.
Results: Four observational studies involving 184 patients were included. Overall mortality of patients treated with anakinra was significantly lower than mortality in the control group (95% CI 0.14-0.48, p<0.0001). Moreover, patients treated with anakinra had a significantly lower risk of need for mechanical ventilation than controls (95% CI 0.250.74, p=0.002). No difference in adverse events and discharge at home with no limitations was observed. The Trial Sequential Analysis z-cumulative line reached the monitoring boundary for benefit and the required sample size.
Conclusions: Administration of anakinra in COVID-19 patients was safe and might be associated with reductions in both mortality and need for mechanical ventilation. Randomized clinical trials are warranted to confirm these findings.
Keywords: Anakinra; COVID-19; Meta-analysis; Mortality; SARS-CoV-2.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
None.
Figures
Comment in
-
Anakinra or tocilizumab for prevention of COVID-19 death? A big dilemma.Eur J Intern Med. 2021 Aug;90:107-108. doi: 10.1016/j.ejim.2021.05.039. Epub 2021 Jun 5. Eur J Intern Med. 2021. PMID: 34158235 Free PMC article. No abstract available.
References
-
- WHO Coronavirus Disease . WHO Coronavirus Disease (COVID-19) Dashboard; 2021. COVID-19) Dashboard. https://covid19.who.int/, accessed February 7, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous