Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 Interferon-Gamma Release Assay
- PMID: 33582369
- PMCID: PMC7879022
- DOI: 10.1016/j.ijid.2021.02.034
Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 Interferon-Gamma Release Assay
Abstract
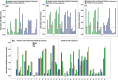
Background: Studies of T-cell immune responses against SARS-CoV-2 are important in understanding the immune status of individuals or populations. Here, we use a simple, cheap, and rapid whole blood stimulation assay - an Interferon-Gamma Release Assay (IGRA) - to study T-cell immunity to SARS-CoV-2 in convalescent COVID-19 patients and in unexposed healthy contacts from Quito, Ecuador.
Methods: Interferon-gamma (INF-γ) production was measured in the heparinized blood of convalescent and unexposed subjects after stimulation for 24 h with the SARS-CoV-2 Spike S1 protein, the Receptor Binding Domain (RBD) protein or the Nucleocapsid (NP) protein, respectively. The presence of IgG-RBD protein antibodies in both study groups was determined with an "in-house" ELISA.
Results: As measured with INF-γ production, 80% of the convalescent COVID-19 patients, all IgG-RBD seropositive, had a strong T-cell response. However, unexpectedly, 44% of unexposed healthy controls, all IgG-RBD seronegative, had a strong virus-specific T-cell response with the COVID-19 IGRA, probably because of prior exposure to common cold-causing coronaviruses or other viral or microbial antigens.
Conclusion and discussion: The high percentage of unexposed healthy subjects with a pre-existing immunity suggests that a part of the Ecuadorian population is likely to have SARS-CoV-2 reactive T-cells. Given that the IGRA technique is simple and can be easily scaled up for investigations where high numbers of patients are needed, this COVID-19 IGRA may serve to determine if the T-cell only response represents protective immunity to SARS-CoV-2 infection in a population-based study.
Keywords: COVID-19; ELISPOT; Interferon-Gamma (IFN-γ) release assay (IGRA); Nucleocapsid protein; Pre-existing immunity; SARS-CoV-2; Spike protein; T cells.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Abate D., Saldan A., Mengoli C., Fiscon M., Silvestre C., Fallico L. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol. 2013;51(8):2501–2507. doi: 10.1128/JCM.00563-13. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

