The effectiveness and adverse effects of D-cycloserine compared with placebo on social and communication skills in individuals with autism spectrum disorder
- PMID: 33583058
- PMCID: PMC8094878
- DOI: 10.1002/14651858.CD013457.pub2
The effectiveness and adverse effects of D-cycloserine compared with placebo on social and communication skills in individuals with autism spectrum disorder
Abstract
Background: Symptoms of autism spectrum disorder (ASD) have been associated, in part, with the dysfunction of N-methyl-D-aspartate (NMDA) glutamate receptors at excitatory synapses and glutamate abnormalities. Medications related to glutamatergic neurotransmission, such as D-cycloserine - which is a partial agonist of the NMDA glutamate receptor - are potential treatment options for the core features of ASD. However, the potential effect of D-cycloserine on the social and communication skills deficits of individuals with ASD has not been thoroughly explored and no systematic reviews of the evidence have been conducted.
Objectives: To assess the efficacy and adverse effects of D-cycloserine compared with placebo for social and communication skills in individuals with ASD.
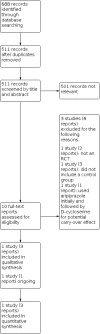
Search methods: In November 2020, we searched CENTRAL, MEDLINE, Embase, six other databases and two trials registers. We also searched the reference lists of relevant publications and contacted the authors of the included study, Minshawi 2016, to identify any additional studies. In addition, we contacted pharmaceutical companies, searched manufacturers' websites and sources of reports of adverse events. SELECTION CRITERIA: All randomised controlled trials (RCTs) of any duration and dose of D-cycloserine, with or without adjunct treatment, compared to placebo in individuals with ASD.
Data collection and analysis: Two review authors independently selected studies for inclusion, extracted relevant data, assessed the risk of bias, graded the certainty of the evidence using the GRADE approach, and analysed and evaluated the data. We provide a narrative report of the findings as only one study is included in this review.
Main results: We included a single RCT (Minshawi 2016) funded by the United States Department of Defense. It was conducted at two sites in the USA: Indiana University School of Medicine and Cincinnati Children's Hospital Medical Centre. In the included study, 67 children with ASD aged between 5 and 11 years were randomised to receive either 10 weeks (10 doses) of (50 mg) D-cycloserine plus social skills training, or placebo plus social skills training. Randomisation was carried out 1:1 between D-cycloserine and placebo arms, and outcome measures were recorded at one-week post-treatment. The 'risk of bias' assessment for the included study was low for five domains and unclear for two domains. The study (67 participants) reported low certainty evidence of little to no difference between the two groups for all outcomes measured at one week post-treatment: social interaction impairment (mean difference (MD) 3.61 (assessed with the Social Responsiveness Scale), 95% confidence interval (CI) -5.60 to 12.82); social communication impairment (MD -1.08 (measured using the inappropriate speech subscale of the Aberrant Behavior Checklist (ABC)), 95% CI -2.34 to 0.18); restricted, repetitive, stereotyped patterns of behaviour (MD 0.12 (measured by the ABC stereotypy subscale), 95% CI -1.71 to 1.95); serious adverse events (risk ratio (RR) 1.11, 95% CI 0.94 to 1.31); non-core symptoms of ASD (RR 0.97 (measured by the Clinical Global Impression-Improvement scale), 95% CI 0.49 to 1.93); and tolerability of D-cycloserine (RR 0.32 (assessed by the number of dropouts), 95% CI 0.01 to 7.68). AUTHORS' CONCLUSIONS: We are unable to conclude with certainty whether D-cycloserine is effective for individuals with ASD. This review included low certainty data from only one study with methodological issues and imprecision. The added value of this review compared to the included study is we assessed the risk of bias and evaluated the certainty of evidence using the GRADE approach. Moreover, if we find new trials in future updates of this review, we could potentially pool the data, which may either strengthen or decrease the evidence for our findings.
Trial registration: ClinicalTrials.gov NCT01086475 NCT00198120.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Swe Zin Aye: none known.
Han Ni: none known.
Htwe Htwe Sein: none known.
San Thidar Mon: none known.
Qishi Zheng: none known.
Yoko Kin Yoke Wong: none known.
Figures












Update of
- doi: 10.1002/14651858.CD013457
References
References to studies included in this review
Minshawi 2016 {published data only}
-
- Minshawi NF, Wink LK, Shaffer R, Plawecki MH, Posey DJ, Liu H, et al. A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in autism spectrum disorders. Molecular Autism 2016;7:2. [DOI: 10.1186/s13229-015-0062-8] [PMC4712595] [PMID: ] - DOI - PMC - PubMed
-
- NCT01086475. D-cycloserine and social skills training in autism spectrum disorders [A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in pervasive developmental disorders]. clinicaltrials.gov/ct2/show/NCT01086475 (first received 10 March 2010).
References to studies excluded from this review
NCT00198107 {published data only}
-
- NCT00198107. Evaluating the effectiveness of Aripiprazole and D-cycloserine to treat symptoms associated with autism [Novel pharmacological strategies in autism]. clinicaltrials.gov/ct2/show/results/NCT00198107 (first received 12 September 2005).
Posey 2004 {published data only}
-
- Posey DJ, Swiezy NB, Kern DL, Sweeten TL, McDougle C. A pilot study of D-cycloserine in the treatment of autistic disorder. Biological Psychiatry 2003;53(8):S170-1. [DOI: 10.1016/S0006-3223(03)00164-1] [Abstract #484] - DOI
Urbano 2014 {published data only}
-
- Urbano M, Okwara L, Manser P, Hartmann K, Deutsch SI. A trial of D-cycloserine to treat the social deficit in older adolescents and young adults with autism spectrum disorders. Journal of Neuropsychiatry and Clinical Neurosciences 2015;27(2):133-8. [DOI: 10.1176/appi.neuropsych.13070155.] [PMID: ] - DOI - PubMed
-
- Urbano M, Okwara L, Manser P, Hartmann K, Herndon A, Deutsch SI. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clinical Neuropharmacology 2014;37(3):69-72. [DOI: 10.1097/WNF.0000000000000033] [PMC4354861] [PMID: ] - DOI - PMC - PubMed
References to ongoing studies
NCT00198120 {published data only (unpublished sought but not used)}
-
- NCT00198120. Safety and effectiveness of D-Cycloserine in children with autism [A randomized controlled trial of D-Cycloserine in autism]. clinicaltrials.gov/ct2/show/study/NCT00198120 (first received 12 September 2005).
Additional references
Abrahams 2008
Aman 1985
-
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency 1985;89(5):485-91. [PMID: ] - PubMed
Attari 2014
Baer 1968
Baio 2018
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report - Surveillance Summaries 2018;67(6):1-23. [DOI: 10.15585/mmwr.ss6706a1] [PMC5919599] [PMID: ] - DOI - PMC - PubMed
Blaylock 2009
Brugha 2011
Bryson 2008
Busner 2007
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
Cola 2020
Constantino 2005
-
- Constantino JN, Gruber CP. The Social Responsiveness Scale. Torrance (CA): Western Psychological Services, 2005.
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 20 November 2020. Available at www.covidence.org.
Crino 2011
Danysz 1998
-
- Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacological Reviews 1998;50(4):597-664. [PMID: ] - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DSM
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 1st edition. Washington (DC): American Psychiatric Association, 1952.
DSM‐5
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington (VA): American Psychiatric Association, 2013.
DSM‐II
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-II). 2nd edition. Washington (DC): American Psychiatric Association, 1968.
DSM‐III
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980.
DSM‐IV
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994.
DSM‐IV‐TR
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition. Washington (DC): American Psychiatric Association, 2000.
Egger 1997
Elsabbagh 2012
Estes 2015
Evans 2010
Ganz 2007
Gargaro 2011
Goff 1999
Goff 2008
Goff 2012
Gould 2017
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 12 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Ha 2015
Heresco‐Levy 2002
-
- Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. American Journal of Psychiatry 2002;159(3):480-2. [DOI: 10.1176/appi.ajp.159.3.480] [PMID: ] - DOI - PubMed
Heys 2018
-
- Heys M, Gibbons F, Haworth E, Medeiros E, Tumbahangphe KM, Wickenden M, et al. The estimated prevalence of autism in school-aged children living in rural Nepal using a population-based screening tool. Journal of Autism and Developmental Disorders 2018;48(10):3483-98. [DOI: 10.1007/s10803-018-3610-1] [PMC6153945] [PMID: ] - DOI - PMC - PubMed
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook.
Hurwitz 2012
Hwang 2013
ICD‐10
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD-10). 10th edition. Geneva (CH): World Health Organization, 2007.
ICD‐11
-
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (ICD-11). 11th edition. Geneva (CH): World Health Organization, 2018.
Kanner 1943
-
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217-50. [psycnet.apa.org/record/1943-03624-001]
Knapp 2009
Leigh 2015
Loomes 2017
Lord 1994
-
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659-85. [PMID: ] - PubMed
Lord 2000
-
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule - Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders 2000;30(3):205-23. [PMID: ] - PubMed
Lyall 2017
Maddox 2017
-
- Maddox BB, Brodkin ES, Calkins ME, Shea K, Mullan K, Hostager J, et al. The accuracy of the ADOS-2 in identifying autism among adults with complex psychiatric conditions. Journal of Autism and Developmental Disorders 2017;47(9):2703-9. [DOI: 10.1007/s10803-017-3188-z] [PMC5813679] [PMID: ] - DOI - PMC - PubMed
Magiati 2014
Matelski 2016
McDougle 2005
-
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. Journal of Clinical Psychiatry 2005;66 Suppl 10:9-18. [PMID: ] - PubMed
Modabbernia 2017
Moher 2009
Moskowitz 2011
NCT01086475
-
- NCT01086475. D-cycloserine and social skills training in autism spectrum disorders [A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in pervasive developmental disorders]. clinicaltrials.gov/ct2/show/NCT01086475 (first received 10 March 2010).
NICE 2013
-
- National Institute for Health and Care Excellence. Autism spectrum disorder in under 19s: support and management. nice.org.uk/guidance/cg170 (accessed 23 September 2019). - PubMed
Padden 2017
-
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. Journal of Developmental and Physical Disabilities 2017;29(4):567-86. [DOI: 10.1007/s10882-017-9547-z] [PMC5502228] [PMID: ] - DOI - PMC - PubMed
Page 2019
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Pisula 2017
Posey 2008
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rodrigues 2014
Rojas 2014
Samadi 2011
Schade 2016
Schiltz 2018
-
- Schiltz HK, McVey AJ, Magnus B, Dolan BK, Willar KS, Pleiss S, et al. Examining the links between challenging behaviors in youth with ASD and parental stress, mental health, and involvement: applying an adaptation of the Family Stress Model to families of youth with ASD. Journal of Autism and Developmental Disorders 2018;48(4):1169-80. [DOI: 10.1007/s10803-017-3446-0] [PMID: ] - DOI - PMC - PubMed
Schünemann 2019
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.. Available from www.training.cochrane.org/handbook.
Sheinin 2001
SIGN 2016
-
- Scottish Intercollegiate Guidelines Network. SIGN 145. Assessment, diagnosis and interventions for autism spectrum disorders. A national clinical guideline. www.sign.ac.uk/assets/sign145.pdf (accessed 23 September 2019).
Sparrow 2005
-
- Sparrow SS, Balla DA, Cicchetti DV, Edgar AD. Vineland Adaptive Behavior Scales. 2nd edition. Circle Pines (MN): American Guidance Service, 2005.
Sturman 2017
Tuchman 2010
White 2009
WHO 2018
-
- World Health Organization. Autistic spectrum disorder. www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (assessed 24 August 2018).
Williams 2012
Williams 2013
Zheng 2016
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

