Onset of Preclinical Alzheimer Disease in Monozygotic Twins
- PMID: 33583080
- PMCID: PMC8251701
- DOI: 10.1002/ana.26048
Onset of Preclinical Alzheimer Disease in Monozygotic Twins
Abstract
Objective: The present work was undertaken to study the genetic contribution to the start of Alzheimer's disease (AD) with amyloid and tau biomarkers in cognitively intact older identical twins.
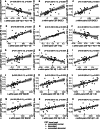
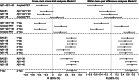
Methods: We studied in 96 monozygotic twin-pairs relationships between amyloid-beta (Aβ) aggregation as measured by the Aβ1-42/1-40 ratio in cerebrospinal fluid (CSF; n = 126) and positron emission tomography (PET, n = 194), and CSF markers for Aβ production (beta-secretase 1, Aβ1-40, and Aβ1-38) and CSF tau. Associations among markers were tested with generalized estimating equations including a random effect for twin status, adjusted for age, gender, and apolipoprotein E ε4 genotype. We used twin analyses to determine relative contributions of genetic and/or environmental factors to AD pathophysiological processes.
Results: Twenty-seven individuals (14%) had an abnormal amyloid PET, and 14 twin-pairs (15%) showed discordant amyloid PET scans. Within twin-pairs, Aβ production markers and total-tau (t-tau) levels strongly correlated (r range = 0.73-0.86, all p < 0.0001), and Aβ aggregation markers and 181-phosphorylated-tau (p-tau) levels correlated moderately strongly (r range = 0.50-0.64, all p < 0.0001). Cross-twin cross-trait analysis showed that Aβ1-38 in one twin correlated with Aβ1-42/1-40 ratios, and t-tau and p-tau levels in their cotwins (r range = -0.28 to 0.58, all p < .007). Within-pair differences in Aβ production markers related to differences in tau levels (r range = 0.49-0.61, all p < 0.0001). Twin discordance analyses suggest that Aβ production and tau levels show coordinated increases in very early AD.
Interpretation: Our results suggest a substantial genetic/shared environmental background contributes to both Aβ and tau increases, suggesting that modulation of environmental risk factors may aid in delaying the onset of AD pathophysiological processes. ANN NEUROL 2021;89:987-1000.
© 2021 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
H.V. is the founder and director of Biomarkable and he is a cofounder of ADx NeuroSciences, where he worked as a consultant through Biomarkable. H.V. has shares in both companies. ADx NeuroSciences provided the enzyme‐linked immunosorbent assays used in this study. The other authors have nothing to report.
Figures


References
-
- Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006;63:168–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

