Microglia regulate synaptic development and plasticity
- PMID: 33583110
- PMCID: PMC8451802
- DOI: 10.1002/dneu.22814
Microglia regulate synaptic development and plasticity
Abstract
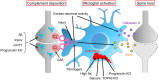
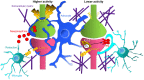
Synapses are fundamental structures of neural circuits that transmit information between neurons. Thus, the process of neural circuit formation via proper synaptic connections shapes the basis of brain functions and animal behavior. Synapses continuously undergo repeated formation and elimination throughout the lifetime of an organism, reflecting the dynamics of neural circuit function. The structural transformation of synapses has been described mainly in relation to neural activity-dependent strengthening and weakening of synaptic functions, that is, functional plasticity of synapses. An increasing number of studies have unveiled the roles of microglia, brain-resident immune cells that survey the brain parenchyma with highly motile processes, in synapse formation and elimination as well as in regulating synaptic function. Over the past 15 years, the molecular mechanisms underlying microglia-dependent regulation of synaptic plasticity have been thoroughly studied, and researchers have reported that the disruption of microglia-dependent regulation causes synaptic dysfunction that leads to brain diseases. In this review, we will broadly introduce studies that report the roles of microglia in synaptic plasticity and the possible underlying molecular mechanisms.
Keywords: microglia; synapse competition; synapse elimination; synapse engulfment; synapse formation.
© 2021 The Authors. Developmental Neurobiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures


References
-
- Adaikkan, C., Middleton, S. J., Marco, A., Pao, P.‐C., Mathys, H., Kim, D.‐W., Gao, F., Young, J. Z., Suk, H.‐J., Boyden, E. S., McHugh, T. J., & Tsai, L.‐H. (2019). Gamma entrainment binds higher‐order brain regions and offers neuroprotection. Neuron, 102, 929–943.e928. 10.1016/j.neuron.2019.04.011 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

