Acid tolerance in early colonizers of oral biofilms
- PMID: 33583397
- PMCID: PMC7883438
- DOI: 10.1186/s12866-021-02089-2
Acid tolerance in early colonizers of oral biofilms
Abstract
Background: In caries, low pH drives selection and enrichment of acidogenic and aciduric bacteria in oral biofilms, and development of acid tolerance in early colonizers is thought to play a key role in this shift. Since previous studies have focussed on planktonic cells, the effect of biofilm growth as well as the role of a salivary pellicle on this process is largely unknown. We explored acid tolerance and acid tolerance response (ATR) induction in biofilm cells of both clinical and laboratory strains of three oral streptococcal species (Streptococcus gordonii, Streptococcus oralis and Streptococcus mutans) as well as two oral species of Actinomyces (A. naeslundii and A. odontolyticus) and examined the role of salivary proteins in acid tolerance development.
Methods: Biofilms were formed on surfaces in Ibidi® mini flow cells with or without a coating of salivary proteins and acid tolerance assessed by exposing them to a challenge known to kill non-acid tolerant cells (pH 3.5 for 30 min) followed by staining with LIVE/DEAD BacLight and confocal scanning laser microscopy. The ability to induce an ATR was assessed by exposing the biofilms to an adaptation pH (pH 5.5) for 2 hours prior to the low pH challenge.
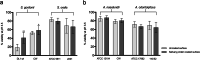
Results: Biofilm formation significantly increased acid tolerance in all the clinical streptococcal strains (P < 0.05) whereas the laboratory strains varied in their response. In biofilms, S. oralis was much more acid tolerant than S. gordonii or S. mutans. A. naeslundii showed a significant increase in acid tolerance in biofilms compared to planktonic cells (P < 0.001) which was not seen for A. odontolyticus. All strains except S. oralis induced an ATR after pre-exposure to pH 5.5 (P < 0.05). The presence of a salivary pellicle enhanced both acid tolerance development and ATR induction in S. gordonii biofilms (P < 0.05) but did not affect the other bacteria to the same extent.
Conclusions: These findings suggest that factors such as surface contact, the presence of a salivary pellicle and sensing of environmental pH can contribute to the development of high levels of acid tolerance amongst early colonizers in oral biofilms which may be important in the initiation of caries.
Keywords: Acid tolerance response; Actinomyces; Pellicle; Salivary proteins; Streptococci.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Plasticity of the Pyruvate Node Modulates Hydrogen Peroxide Production and Acid Tolerance in Multiple Oral Streptococci.Appl Environ Microbiol. 2018 Jan 2;84(2):e01697-17. doi: 10.1128/AEM.01697-17. Print 2018 Jan 15. Appl Environ Microbiol. 2018. PMID: 29079629 Free PMC article.
-
Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source.Infect Immun. 2001 Sep;69(9):5794-804. doi: 10.1128/IAI.69.9.5794-5804.2001. Infect Immun. 2001. PMID: 11500457 Free PMC article.
-
Acid tolerance of biofilm cells of Streptococcus mutans.Appl Environ Microbiol. 2007 Sep;73(17):5633-8. doi: 10.1128/AEM.01049-07. Epub 2007 Jul 13. Appl Environ Microbiol. 2007. PMID: 17630302 Free PMC article.
-
The role of bacteria in the caries process: ecological perspectives.J Dent Res. 2011 Mar;90(3):294-303. doi: 10.1177/0022034510379602. Epub 2010 Oct 5. J Dent Res. 2011. PMID: 20924061 Review.
-
Does assessment of microbial composition of plaque/saliva allow for diagnosis of disease activity of individuals?Community Dent Oral Epidemiol. 1997 Feb;25(1):76-81. doi: 10.1111/j.1600-0528.1997.tb00902.x. Community Dent Oral Epidemiol. 1997. PMID: 9088695 Review.
Cited by
-
Characteristics of salivary microbiota in children with obstructive sleep apnea: A prospective study with polysomnography.Front Cell Infect Microbiol. 2022 Aug 29;12:945284. doi: 10.3389/fcimb.2022.945284. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36105146 Free PMC article.
-
[Effect of Lipoteichoic Acid Synthesis-Related Gene dltD on Acid Tolerance of Highly Cariogenic Strains of Streptococcus mutans].Sichuan Da Xue Xue Bao Yi Xue Ban. 2022 Mar;53(2):235-241. doi: 10.12182/20220360102. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35332723 Free PMC article. Chinese.
-
Oral biofilm composition and phenotype in caries-active and caries-free children.Front Oral Health. 2024 Oct 22;5:1475361. doi: 10.3389/froh.2024.1475361. eCollection 2024. Front Oral Health. 2024. PMID: 39502319 Free PMC article.
-
Effects of glucose and lactate on Streptococcus mutans abundance in a novel multispecies oral biofilm model.Microbiol Spectr. 2024 Apr 2;12(4):e0371323. doi: 10.1128/spectrum.03713-23. Epub 2024 Feb 20. Microbiol Spectr. 2024. PMID: 38376204 Free PMC article.
-
New Preventive Strategy against Oral Biofilm Formation in Caries-Active Children: An In Vitro Study.Antibiotics (Basel). 2023 Jul 31;12(8):1263. doi: 10.3390/antibiotics12081263. Antibiotics (Basel). 2023. PMID: 37627682 Free PMC article.
References
-
- Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44(Suppl 18):S5–s11. doi: 10.1111/jcpe.12682. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

