EEG measures of sensorimotor processing and their development are abnormal in children with isolated dystonia and dystonic cerebral palsy
- PMID: 33583764
- PMCID: PMC8044718
- DOI: 10.1016/j.nicl.2021.102569
EEG measures of sensorimotor processing and their development are abnormal in children with isolated dystonia and dystonic cerebral palsy
Abstract
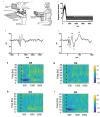
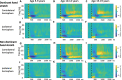
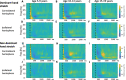
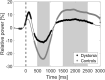
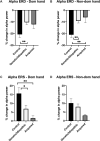
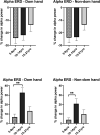
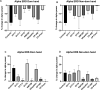
Dystonia is a disorder of sensorimotor integration associated with abnormal oscillatory activity within the basal ganglia-thalamo-cortical networks. Event-related changes in spectral EEG activity reflect cortical processing but are sparsely investigated in relation to sensorimotor processing in dystonia. This study investigates modulation of sensorimotor cortex EEG activity in response to a proprioceptive stimulus in children with dystonia and dystonic cerebral palsy (CP). Proprioceptive stimuli, comprising brief stretches of the wrist flexors, were delivered via a robotic wrist interface to 30 young people with dystonia (20 isolated genetic/idiopathic and 10 dystonic CP) and 22 controls (mean age 12.7 years). Scalp EEG was recorded using the 10-20 international system and the relative change in post-stimulus power with respect to baseline was calculated for the alpha (8-12 Hz) and beta (14-30 Hz) frequency bands. A clear developmental profile in event-related spectral changes was seen in controls. Controls showed a prominent early alpha/mu band event-related desynchronisation (ERD) followed by an event-related synchronisation (ERS) over the contralateral sensorimotor cortex following movement of either hand. The alpha ERD was significantly smaller in the dystonia groups for both dominant and non-dominant hand movement (ANCOVA across the 3 groups with age as covariate: dominant hand F(2,47) = 4.45 p = 0.017; non-dominant hand F(2,42) = 9.397 p < 0.001. Alpha ERS was significantly smaller in dystonia for the dominant hand (ANCOVA F(2,47) = 7.786 p = 0.001). There was no significant difference in ERD or ERS between genetic/idiopathic dystonia and dystonic CP. CONCLUSION: Modulation of alpha/mu activity by a proprioceptive stimulus is reduced in dystonia, demonstrating a developmental abnormality of sensorimotor processing which is common to isolated genetic/idiopathic and acquired dystonia/dystonic CP.
Keywords: Dystonia; Dystonic cerebral palsy; Event-related desynchronisation; Event-related synchronisation; Mu modulation; Sensorimotor integration.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Jean-Pierre Lin has received educational support and consultancy fees from Medtronic Ltd.
Peter Brown has also received consultancy fees from Medtronic Ltd.
Figures
References
-
- Tinazzi M., Priori A., Bertolasi L., Frasson E., Mauguiere F., Fiaschi A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain. 2000;123(Pt 1):42–50. - PubMed
-
- Frasson E., Priori A., Bertolasi L., Mauguiere F., Fiaschi A., Tinazzi M. Somatosensory disinhibition in dystonia. Mov. Disord. 2001;16:674–682. - PubMed
-
- Nelson A.J., Blake D.T., Chen R. Digit-specific aberrations in the primary somatosensory cortex in Writer's cramp. Ann. Neurol. 2009;66:146–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

