Screening Trimethoprim Primary Metabolites for Covalent Binding to Albumin
- PMID: 33584083
- PMCID: PMC7879966
- DOI: 10.1007/s00044-020-02570-z
Screening Trimethoprim Primary Metabolites for Covalent Binding to Albumin
Abstract
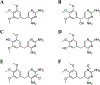
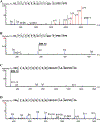
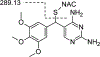
Modification of endogenous proteins by drugs and drug metabolites are thought to be a cause of idiosyncratic adverse drug reactions (IADRs). Trimethoprim (TMP) is a commonly prescribed antibiotic that has been implicated in IADRs; however, there is no known mechanism by which this drug or its metabolites modify proteins. This study describes the results of screening trimethoprim and its primary metabolites for the ability to covalently modify human serum albumin (HSA). The first step of the screen was in vitro reactions of the compounds with HSA followed by western blotting with antisera specific to drug-modified proteins. Compounds with positive signal in the western blot were then screened using an untargeted peptide profiling method to discover modified peptides. This strategy identified two sites in HSA that are modified by incubation with a TMP metabolite, α-hydroxy trimethoprim (Cα-OH-TMP).
Keywords: Trimethoprim; adverse drug reaction; metabolism; protein adduct.
Conflict of interest statement
Conflict of Interest: The authors declare they have no conflict of interest.
Figures

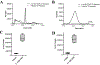
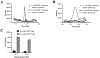
References
-
- Aldini G, Gamberoni L, Orioli M, Beretta G, Regazzoni L, Maffei Facino R, Carini M (2006) Mass spectrometric characterization of covalent modification of human serum albumin by 4-hydroxy-trans-2-nonenal. Journal of mass spectrometry : JMS 41:1149–1161 - PubMed
-
- Cho T, Uetrecht J (2017) How Reactive Metabolites Induce an Immune Response That Sometimes Leads to an Idiosyncratic Drug Reaction. Chem Res Toxicol 30:295–314 - PubMed
-
- Damsten MC, de Vlieger JS, Niessen WM, Irth H, Vermeulen NP, Commandeur JN (2008) Trimethoprim: novel reactive intermediates and bioactivation pathways by cytochrome p450s. Chem Res Toxicol 21:2181–2187 - PubMed
-
- Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA (2004) Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem Res Toxicol 17:3–16 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
